DOI: 10.12809/hkmj144403
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Extrapulmonary involvement associated with Mycoplasma pneumoniae infection
T Liong, FHKCP, FHKAM (Medicine)1;
KL Lee, FHKCP, FHKAM (Medicine)1;
YS Poon, FHKCP, FHKAM (Medicine)1;
SY Lam, FHKCP, FHKAM (Medicine)1;
KM Kwok, MRCP1;
WF Ng, FHKAM (Pathology)2;
TL Lam, FHKAM (Pathology)2;
KI Law, FHKCP, FHKAM (Medicine)1
1 Intensive Care Unit, United Christian Hospital, Kwun Tong, Hong Kong
2 Department of Pathology, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr KI Law (lawki@ha.org.hk)
Abstract
Mycoplasma pneumoniae infection usually presents
with upper and lower respiratory tract infection.
Extrapulmonary involvement is not uncommon,
however. We report two cases of predominantly
extrapulmonary manifestations of Mycoplasma
pneumoniae infection without significant
pulmonary involvement. Both cases were diagnosed
by serology. These cases illustrate the diversity of
clinical presentations of Mycoplasma pneumoniae
infection. Clinicians should maintain a high index
of suspicion.
Case reports
Case 1
A 20-year-old man who worked as a car mechanic
and enjoyed good health presented to United
Christian Hospital in Hong Kong in August 2013 after
collapsing suddenly at work. Ventricular arrhythmia
was detected by ambulance men and defibrillation
was performed 3 times with an automated external
defibrillator. He was then transferred to the
emergency department of the same hospital where
he was observed to have persistent ventricular
tachycardia and fibrillation. Advanced cardiac life
support was continued, repeated defibrillation was
performed and spontaneous circulation was restored
53 minutes later when he was then intubated.
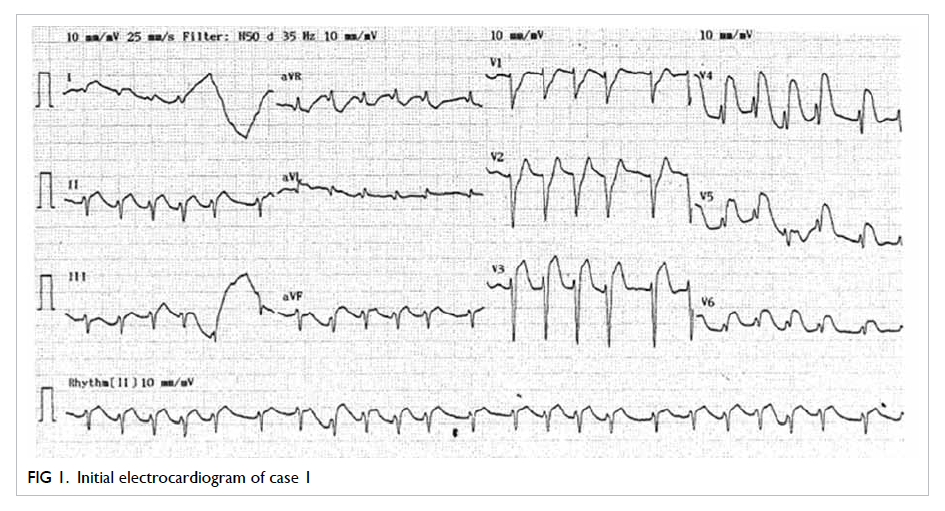
Initial electrocardiogram after successful
resuscitation showed diffuse ST elevation over
the chest leads (Fig 1). Echocardiogram revealed poor left ventricular systolic function with global
hypokinesia. Ad-hoc cardiac catheterisation was
carried out. There was no coronary lesion and the
patient was transferred to the intensive care unit
(ICU) for therapeutic hypothermia.
On arrival in ICU, the patient had a high fever
up to 39.7°C. Blood pressure was normal on low-dose
inotropes and pulse rate was 140 beats/minute.
Glasgow Coma Scale score was 2 for eye opening, 1
for verbal response, and 1 for motor response. His
pupils were equal and reactive, and bilateral plantar
reflexes were equivocal. Frothy sputum was evident
from the endotracheal tube but physical examination
was otherwise unremarkable.
Initial blood tests revealed elevated white
cell count (WCC) of 25.1 x 109 /L (reference range
[RR], 3.9-9.3 x 109 /L), neutrophilia of 21.6 x 109 /L
(RR, 1.8-6.2 x 109 /L), and normal lymphocyte count
of 2.9 x 109 /L (RR, 1.0-3.2 x 109 /L). Haemoglobin
level and platelet count were normal. He had mild
renal and liver impairment with urea 8.2 mmol/L
(RR, 2.8-8.1 mmol/L), and creatinine 138 µmol/L (RR,
62-106 µmol/L). Sodium and potassium levels were
normal and alanine transferase (ALT) was 152 IU/L
(reference level [RL], <41 IU/L). Creatinine kinase
was 6439 IU/L (RR, 39-308 IU/L), and troponin I
was 12 886 ng/L (RL, ≤14 ng/L). Initial urine toxic
screening was negative. His first chest X-ray (CXR)
showed right upper lobe consolidation, but repeated
CXR the next morning showed almost complete
resolution. Initial computed tomography (CT) of the
brain was unremarkable.
Amoxicillin/clavulanate was started to cover
aspiration pneumonia. Therapeutic hypothermia
for neuroprotection and continuous veno-venous
haemofiltration for acute kidney injury were
commenced. On day 4 the patient developed
convulsions and repeated CT brain showed diffuse
cerebral oedema. An electroencephalogram showed
diffuse encephalopathy compatible with severe
hypoxic-ischaemic insult. The patient remained in a
vegetative state and a tracheostomy was performed.
He had repeated episodes of ventilator-associated
pneumonia that were unresponsive to multiple
regimens of antibiotics and finally succumbed on
day 24 of admission.
Serology for viral studies was all negative.
Nonetheless, paired serology for Mycoplasma
pneumoniae on 26 August 2013 and 4 September
2013 showed a 4-fold decrease in antibody titres from
1:160 to 1:40, suggestive of recent M pneumoniae
infection. Postmortem examination showed diffuse
lymphocytic infiltrates and extensive myocardial
necrosis within myocardial fibres (Fig 2). Reversetranscriptase polymerase chain reaction (RT-PCR)
for M pneumoniae on bilateral lung tissue and myocardium were negative.
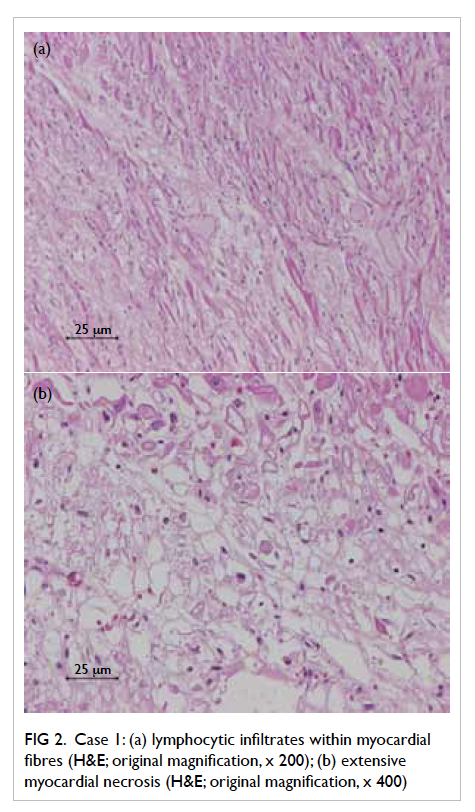
Figure 2. Case 1: (a) lymphocytic infiltrates within myocardial fibres (H&E; original magnification, x 200); (b) extensive myocardial necrosis (H&E; original magnification, x 400)
Case 2
A 57-year-old security guard with a history of
infrequent asthma attacks but no regular follow-up
attended the emergency department of United
Christian Hospital in September 2013 with fever,
chills, and rigors. He also complained of a new-onset
maculopapular rash over the trunk and limbs that
developed 1 day prior to admission. He volunteered
that he was constantly exposed to insect bites due
to the close proximity of rural areas to his working
environment.
After admission to the medical ward, he
developed a high fever of 40.3°C. Blood pressure
was normal and pulse rate was 110 beats/minute.
Oxygen saturation could be maintained on low-flow
oxygen. Physical examination of the cardiovascular
system, chest, and abdomen was normal but a
maculopapular rash compatible with erythema
multiforme over the back, lower abdomen, and
bilateral lower limbs was observed. No eschar could
be seen.
Initial investigations revealed the following:
elevated WCC at 22.1 x 109 /L, neutrophilia of
21 x 109 /L, and lymphopenia of 0.2 x 109 /L. The
haemoglobin level was 117 g/L (RR, 135-173
g/L) and platelet count was 79 x 109 /L (RR,
160-420 x 109 /L). The clotting profile was mildly
deranged, with international normalised ratio of
1.3. He also had mild renal and liver impairment:
creatinine 111 µmol/L, ALT 74 IU/L, and aspartate
aminotransferase 101 IU/L. Initial CXR did not
reveal any consolidative changes.
Amoxicillin/clavulanate was commenced but
the patient’s condition deteriorated with development
of septic shock that required high-dose inotropes,
type I respiratory failure requiring high-flow oxygen,
acute kidney injury, and disseminated intravascular
coagulopathy despite change in antibiotic therapy to
piperacillin/tazobactam and azithromycin soon after
admission. He was transferred to ICU on day 3 of
admission and required intubation due to aspiration.
Antibiotics were changed to piperacillin/tazobactam
and doxycycline. His condition gradually improved
and he was successfully extubated and weaned off all
inotropes. The skin rash also resolved spontaneously
and repeated biochemistry tests revealed complete
resolution of renal and liver derangement.
Skin biopsy was not performed. The Widal test
and Weil-Felix tests were all negative. Paired serology
of M pneumoniae showed a 4-fold rise in antibody
titres from 1:<10 to 1:40, with confirmation of M
pneumoniae infection. Viral serology for rubella and
salmonella was not increased.
Discussion
We report two cases of M pneumoniae infection that
presented with extrapulmonary symptoms and no
significant respiratory involvement. Mycoplasma
pneumoniae usually infects the upper and lower
respiratory tract. Up to 50% of patients develop
only mild upper respiratory tract symptoms such as
cough, sore throat, malaise, and only 3% to 10% of
patients develop pneumonia.1
Approximately 25% of patients infected with M
pneumoniae develop extrapulmonary complications.
This can happen before, during, or after the onset
of or even in the absence of respiratory symptoms.1
An autoimmune reaction has been suggested as
the pathogenesis of many of these extrapulmonary
complications.1 The presence of organisms at an
extrapulmonary site, however, suggests that direct
invasion is also an important mechanism.2 3
The extrapulmonary manifestations associated
with M pneumoniae may be neurological, cardiac,
dermatological, musculoskeletal, haematological,
and gastro-intestinal.4 Of these, neurological and
dermatological symptoms are recognised as among
the most common extrapulmonary manifestations
of M pneumoniae infection.1 4
A wide range of dermatological manifestations
has been described in patients with M pneumoniae
infection. Mild symptoms include a maculopapular
or vesicular rash.5 Erythema multiforme and Stevens-Johnson syndrome have a strong association with M pneumoniae infection.4 6 7 8
The patient in case 2 presented with sepsis and
erythema multiforme. He was not prescribed any
medication associated with erythema multiforme.
Since a list of infective causes of erythema
multiforme had been excluded by serology tests,
his skin manifestation was most likely due to M
pneumoniae infection.
Cardiac involvement is regarded as an
uncommon complication of M pneumoniae infection.1 Pericarditis, myocarditis or pericardial effusion with or without tamponade effect have been
described.1 It is more commonly found in adults than
in paediatric patients.9 Fortunately, the outcome is
generally good. Only a minority of patients had long-term sequelae and mortality is rare.10
Myocarditis associated with M pneumoniae
infection that presents with ventricular arrhythmia
as in case 1 is rare. We excluded other common
causes of ventricular arrhythmia by initial blood
tests, toxic screening, cardiac catheterisation, and
CT brain. A 4-fold decrease in antibody titre of paired
sera confirmed recent M pneumoniae infection.
Autopsy results showed lymphocytic myocarditis.
It is plausible, although not confirmative, that M
pneumonia–associated myocarditis due to direct
invasion of M pneumoniae cannot be shown by
RT-PCR. Other differential diagnoses of lymphocytic
myocarditis such as viral myocarditis were excluded
by viral serology.
In addition to the diverse clinical
manifestations, clinicians should also be aware of
widespread macrolide resistance of M pneumoniae
in Asia, including Hong Kong.11 High rates of
macrolide resistance of up to 70% to 80% have been
reported in China and Japan.12 13 14 Prompt adjustment
of antibiotics to cover atypical pathogens is essential
to successful treatment, as in our second patient.
Consideration of alternatives such as doxycycline or
fluoroquinolones as empirical treatment of atypical
pathogens in areas with high rates of resistance may
be appropriate.14
In conclusion, although M pneumoniae most
commonly presents with respiratory tract symptoms,
extrapulmonary manifestations are not uncommon.
Clinicians should be aware of variable clinical
presentations of M pneumoniae and macrolide
resistance in our locality.
References
1. Waites KB, Talkington DF. Mycoplasma pneumoniae
and its role as a human pathogen. Clin Microbiol Rev
2004;17:697-728. Crossref
2. Kasahara I, Otsubo Y, Yanase T, Oshima H, Ichimaru
H, Nakamura M. Isolation and characterization of
Mycoplasma pneumoniae from cerebrospinal fluid of a
patient with pneumonia and meningoencephalitis. J Infect
Dis 1985;152:823-5. Crossref
3. Saïd MH, Layani MP, Colon S, Faraj G, Glastre C, Cochat
P. Mycoplasma pneumoniae–associated nephritis in
children. Pediatr Nephrol 1999;13:39-44. Crossref
4. Sánchez-Vargas FM, Gómez-Duarte OG. Mycoplasma
pneumoniae—an emerging extra-pulmonary pathogen.
Clin Microbiol Infect 2008;14:105-17. Crossref
5. Baum SG. Mycoplasma pneumoniae infection in
adults. Available from: http://www.uptodate.com/contents/mycoplasma-pneumoniae-infection-in-adults?
source=search_result&search=Mycoplasma+pneumoniae+infection+in+adults.&selectedTitle=1%7E116.
Accessed Aug 2015.
6. Vanfleteren I, Van Gysel D, De Brandt C. Stevens-Johnson
syndrome: a diagnostic challenge in the absence of skin
lesions. Pediatr Dermatol 2003;20:52-6. Crossref
7. Vargas-Hitos JA, Manzano-Gamero MV, Jiménez-Alonso
J. Erythema multiforme associated with Mycoplasma pneumoniae. Infection 2014;42:797-8. Crossref
8. Rock N, Belli D, Bajwa N. Erythema bullous multiforme:
a complication of Mycoplasma pneumoniae infection. J Pediatr 2014;164:421. Crossref
9. Formosa GM, Bailey M, Barbara C, Muscat C, Grech
V. Mycoplasma pneumonia—an unusual cause of acute
myocarditis in childhood. Images Paediatr Cardiol
2006;8:7-10.
10. Paz A, Potasman I. Mycoplasma-associated carditis. Case
reports and review. Cardiology 2002;97:83-8. Crossref
11. Ho PL, Wong SY, editors. Reducing bacterial resistance with
IMPACT—Interhospital Multi-disciplinary Programme on Antimicrobial ChemoTherapy. 4th ed. Hong Kong SAR: Centre for Health Protection; 2012.
12. Yin YD, Cao B, Wang H, et al. Survey of macrolide
resistance in Mycoplasma pneumoniae in adult patients with community-acquired pneumonia in Beijing, China [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi 2013;36:954-8.
13. Eshaghi A, Memari N, Tang P, et al. Macrolide-resistant
Mycoplasma pneumoniae in humans, Ontario, Canada,
2010-2011. Emerg Infect Dis 2013;19(9). doi: 10.3201/eid1909.121466. Crossref
14. Okada T, Morozumi M, Tajima T, et al. Rapid effectiveness
of minocycline or doxycycline against macrolide-resistant
Mycoplasma pneumoniae infection in a 2011 outbreak
among Japanese children. Clin Infect Dis 2012;55:1642-9. Crossref