DOI: 10.12809/hkmj144357
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Churg-Strauss syndrome from an orthopaedic perspective
KL Kung, MB, BS;
PK Yee, MB, ChB, FHKCOS
Department of Orthopaedics and Traumatology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr KL Kung (lepetitcarmen@gmail.com)
Abstract
Churg-Strauss syndrome, which has been frequently
described by physicians in the literature, is a
small and medium-sized vessel systemic vasculitis
typically associated with asthma, lung infiltrates,
and hypereosinophilia. We report a case of Churg-Strauss syndrome with presenting symptoms of
bilateral lower limb weakness and numbness only.
The patient was admitted to an orthopaedic ward
for management and a final diagnosis was reached
following sural nerve biopsy. The patient’s symptoms
responded promptly to steroid treatment and she
was able to walk with a stick 3 weeks following
admission. This report emphasises the need to be
aware of this syndrome when managing patients
with neurological deficit in order to achieve prompt
diagnosis and treatment.
Introduction
Churg-Strauss syndrome (CSS) is a small and
medium-sized vessel vasculitis that can affect
different organs. The usual presentation is sub-optimised
control of asthma together with
involvement of other organs such as the heart,
skin, and nervous system. It seldom presents with
isolated neurological symptoms and has thus far
not been reported from an orthopaedic aspect. We
report a case of CSS in a patient who presented with
neurological symptoms and who was admitted to the
orthopaedic ward via casualty because of lower limb
weakness and numbness.
Case report
A 66-year-old Chinese woman was admitted to the
orthopaedic ward for 2 weeks in May 2013 because
of weakness and numbness in both lower limbs.
She had a more than 10 years’ history of asthma
that was controlled with an inhaled bronchodilator
and oral theophylline and terbutaline. The patient
was prescribed an oral steroid intermittently for
acute control. From January 2012 until the current
admission, she had also been taking a leukotriene
receptor antagonist (montelukast).
On admission, the patient complained of severe
dysesthesia over both lower limbs, mainly below
knee level. There was mild left proximal thigh pain.
She had no low back pain and denied a history of
recent trauma. The symptoms rendered the patient
unable to walk.
Upon physical examination, she was
afebrile, and cardiopulmonary and dermatological
examination was unremarkable. Neurological
examination revealed decreased sensation over both
lower limbs in a glove and stocking distribution.
Power of the muscles supplied by the peroneal
and tibial nerves was grade 4 according to Medical
Research Council grading system. Reflexes were
diminished over both lower limbs. Per rectal
examination revealed normal anal tone.
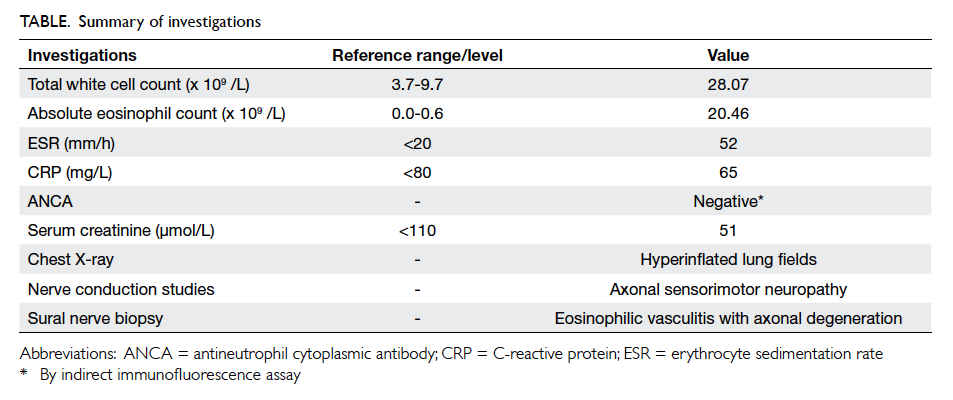
Blood tests revealed elevated white cell count
to 28.07 x 109 /L with a predominance of eosinophils
(20.46 x 109 /L). C-reactive protein was 65 mg/L.
Erythrocyte sedimentation rate was 52 mm/h.
Serum calcium, phosphate, alkaline phosphatase
and creatine kinase level were normal. Sepsis
workup including blood culture and urine culture
were negative (Table).
Radiography of the lumbar spine revealed
grade-one spondylolisthesis at the lumbar 4 and 5
level. Radiography of the pelvis was unremarkable.
Chest radiography showed slightly hyperinflated
lung. Subsequent magnetic resonance imaging
with contrast of the lumbar spine and pelvis was
performed in view of the radiographic findings of
the lumbar spine and the neurological deficit in the
lower limbs. There was neither evidence of nerve
root and cord compression nor infection around the
lumbar spine.
Nerve conduction study showed absence of
the compound muscle action potential (CMAP) of
the right peroneal nerve recorded at the extensor
digitorum brevis muscle. The CMAP was also
decreased over bilateral tibialis anterior muscles.
These results were suggestive of an axonal type of
motor neuropathy over bilateral peroneal nerves and
a sensory type of axonal neuropathy over the right
lower limb. There was no involvement of the upper
limbs.
Left sural nerve biopsy revealed epineurial
extravascular eosinophils and vasculitis associated
with axonal degeneration (Fig). There was one small epineurial artery infiltrated by eosinophils,
polymorphs, and lymphocytes. Vague granuloma
were present in the vascular wall. These features
were consistent with CSS based on the American
College of Rheumatology Classification criteria.1
The Table summarises the investigations.
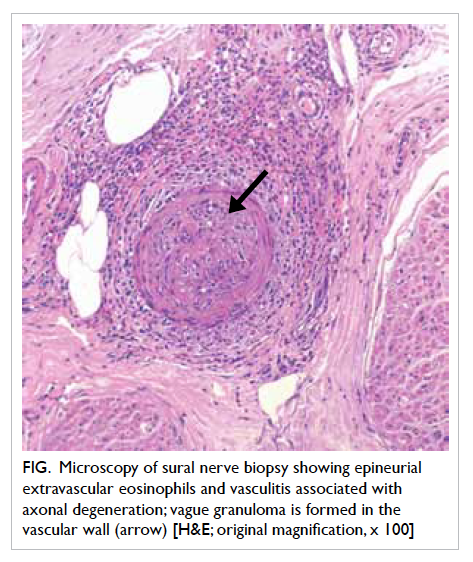
Figure. Microscopy of sural nerve biopsy showing epineurial extravascular eosinophils and vasculitis associated with axonal degeneration; vague granuloma is formed in the vascular wall (arrow) [H&E; original magnification, x 100]
A rheumatologist was consulted who
diagnosed CSS with peripheral neuropathy.
Montelukast was discontinued in view of the
possible association of the CSS. Oral prednisolone
40 mg daily was prescribed to the patient and an oral
bisphosphonate was given to prevent osteoporosis.
Distal lower limb power improved to grade 5 shortly
following steroid treatment and there was marked
improvement in pain and numbness. The eosinophil
count and elevated inflammatory markers reduced
rapidly over 6 days. The patient was discharged from
the orthopaedic ward 2 weeks after treatment and
was able to walk with a stick. The patient has been
referred to a day hospital for further rehabilitation.
Discussion
Churg-Strauss syndrome is an entity frequently
described by rheumatologists in the literature.2 It has
seldom been reported by an orthopaedic surgeon.
This case illustrates that common symptoms such as
pain and numbness, frequently encountered when
treating orthopaedic patients, may not be necessarily
due to an orthopaedic problem. It may be a medical
disease that requires prompt and specific treatment.
Allergic granulomatosis angiitis, or CSS, is
a type of antineutrophil cytoplasmic antibody–associated small-vessel systemic vasculitis. Other
diseases under the same group include microscopic
polyangiitis and granulomatosis with polyangiitis,
formerly known as Wegener’s granulomatosis.3 It
was first described in 1951 by Dr Jacob Churg and
Dr Lotte Strauss at Mount Sinai Hospital and is
characterised by eosinophilic vasculitis that affects
the small and medium-sized vessels. It describes
the clinical symptoms of the pathological entity
allergic angiitis and granulomatosis. The American
College of Rheumatology has recommended that
diagnosis of the syndrome is considered when four
of the following features are present: (1) asthma, (2)
eosinophils constituting more than 10% of the white
cell count, (3) neuropathy, (4) non-fixed pulmonary
infiltrates on radiography, (5) extravascular
granulomas, and (6) abnormalities of the paranasal
sinuses.1 The presence of four or more criteria yields
a diagnostic sensitivity of 85% and a specificity of
99%.1
Despite variable clinical manifestations,
pathological findings will include necrotising
eosinophilic vasculitis that may result from
endothelial cell adhesion and leukocyte activation,
with subsequent necrotising vasculitis in several
organ systems.
The pathogenesis of the syndrome is unclear,
but it has been shown that HLA-DRB3 is a genetic
risk factor for development. There have been reports
about the strong association between CSS and the
use of a leukotriene receptor antagonist (LTRA)
such as montelukast. The mechanism by which
LTRAs might cause eosinophilic vasculitis remains
unclear, however. It is known that LTRAs do not
inhibit leukotriene B4 (LTB4), which is a powerful
chemoattractant for eosinophils. This could lead
to increased plasma levels of LTB4 and trigger
eosinophilic inflammation. Nevertheless, LTRA
has a somewhat causative role in the development
of CSS. A controlled study with a large number of
patients is required to verify this conclusion.
The clinical presentation is variable. Some
people have only mild symptoms, while others
experience severe or life-threatening complications.
There are three stages of CSS, but not every patient
will develop all three phases or in the same order.
They include:
(1) Allergic stage: the first stage of the syndrome in which the patient develops a number of allergic reactions including asthma, hay fever, and sinusitis.
(2) Eosinophilic stage: hypereosinophilia is found in the blood or tissue causing serious local damage. The lungs and digestive tract are most often involved. The symptoms may be relapsing and remitting and last for months or years.
(3) Vasculitic stage: the hallmark of this stage is blood vessel inflammation. The blood vessels are narrowed by inflammation and blood flow to tissue is jeopardised. During this phase, a feeling of general ill-health along with unintended weight loss, lymphadenopathy, weakness, and fatigue may occur.
(1) Allergic stage: the first stage of the syndrome in which the patient develops a number of allergic reactions including asthma, hay fever, and sinusitis.
(2) Eosinophilic stage: hypereosinophilia is found in the blood or tissue causing serious local damage. The lungs and digestive tract are most often involved. The symptoms may be relapsing and remitting and last for months or years.
(3) Vasculitic stage: the hallmark of this stage is blood vessel inflammation. The blood vessels are narrowed by inflammation and blood flow to tissue is jeopardised. During this phase, a feeling of general ill-health along with unintended weight loss, lymphadenopathy, weakness, and fatigue may occur.
Asthma is the central feature of CSS and
precedes the systemic manifestations in almost
all cases. Upper airway findings can include
sinusitis, allergic rhinitis, and nasal polyps. Cardiac
involvement represents the major cause of mortality.
Granulomatous infiltration of the myocardium and
coronary vessel vasculitis are the most common
lesions. Congestive heart failure may develop
rapidly and is often responsible for the mortality.
Gastro-intestinal involvement such as abdominal
pain, diarrhoea, and bleeding may also occur.
Bowel perforation is the most severe manifestation
and is one of the major causes of death. The
glomerular lesion that typifies CSS is focal segmental
glomerulonephritis with necrotising crescents. Its
involvement is one of the poor prognostic factors.
Arthralgias are frequent but arthritis with local
inflammatory findings is rare, and joint deformity
and radiographic erosions are usually not seen.
Large articular joints are affected more than small
joints. The symptoms usually regress quickly with
treatment. Skin involvement occurs in 50% to 68%
of patients and reflects the predilection for small
vessels. Lesions are red or violaceous, and occur
primarily on the scalp and the limbs or hands and
feet. They are often bilateral and symmetrical.4
Peripheral neuropathy, as in our case, is
common in patients with CSS (65-75%).5 6 The
initial symptoms of neuropathy usually include
an acute onset of tingling or painful paresthesia in
the extremities. These symptoms predominantly
occur in the distal portions of the extremities,
particularly the lower limbs. In the initial phase, the
distribution of sensory impairment usually takes
the form of a mononeuritis multiplex pattern. As
the disease progresses, it eventually evolves into
a polyneuropathic pattern. Muscle weakness, to a
variable extent and degree of asymmetry, may also
be evident. There will be decreased or absent deep
tendon reflexes corresponding to the distribution of
nerves involved. Electromyography reveals axonal
nerve involvement and often detects more extensive
involvement than the clinical symptoms would
indicate. With treatment, mononeuritis multiplex
regresses progressively and patients may recover
without sequelae. Prompt diagnosis and treatment
are of paramount importance in managing CSS with
neuropathy. If medical treatment is delayed, atrophy
and weakness of the limbs may be irreversible
and will require additional rehabilitation therapy
including strengthening exercises, balance training,
and ambulation training.
In the treatment of CSS, as with other forms of
vasculitis, the symptoms respond to corticosteroids
or other immunosuppressive drugs, eg
cyclophosphamide and methotrexate.7 If refractory
to these agents, intravenous immunoglobulin is
the treatment of choice. Leukocytosis, eosinophilia,
and raised erythrocyte sedimentation rates usually
respond promptly after administration of these
agents. Neuropathic pain also decreases rapidly
in 85% of patients. There is speculation that the
initial clinical course and the degree of systemic
inflammatory involvement may influence long-term
functional prognosis. Recent trials of treatment
include immunomodulators such as rituximab,
an anti-CD20 monoclonal antibody, and tumour
necrosis factor–alpha.8
In this article, we have reviewed the clinical
features, diagnostic criteria, and treatment options
of CSS. In middle-aged or elderly patients, lower
limb numbness and pain are frequently attributed
to the degenerative spine with stenosis or nerve root
compression. It is tempting to conclude that a patient
has spinal stenosis or lumbar spondylosis in the
presence of degenerative lumbar spine radiographs.
This case highlights the need for clinicians to be
vigilant for these manifestations in addition to the
various diagnoses of spinal pathology when a patient
presents with limb numbness and weakness together
with a history of asthma and raised eosinophil count.
Because signs and symptoms are both numerous and
at times unassuming, it is notoriously difficult to
diagnose CSS at the very initial phase. Nonetheless,
significant neuropathic involvement may be
prevented if a patient receives adequate therapy to
induce remission of disease and prevent relapse.
With treatment, most of the symptoms in any of
the three phases can be relieved. We would like to
raise the awareness of such an entity in treating
patients with neuropathy. Close collaboration with
rheumatologists in treating such a complex illness,
including prompt diagnosis by performing nerve
biopsy by an orthopaedic surgeon and prompt
medical treatment by a rheumatologist, is the key to
successful management.
References
1. Masi AT, Hunder GG, Lie TT, et al. The American College
of Rheumatology 1990 criteria for the classification of
Churg-Strauss syndrome (allergic granulomatosis and
angiitis). Arthritis Rheum 1990;33:1094-100. Crossref
2. Churg J, Strauss L. Allergic granulomatosis, allergic rhinitis,
and periarthritis nodosa. Am J Pathol 1951;27:277-301.
3. Falk RJ, Gross WL, Guillvein L, et al. Granulomatosis
with polyangiitis (Wegener’s): An alternative name for
Wegener’s granulomatosis. Arthritis Rheum 2011;63:863-4. Crossref
4. Guillevin L, Pagnoux C, Mouthon L. Churg-Strauss
syndrome. Semin Respir Crit Care Med 2004;25:535-45. Crossref
5. Sehgal M, Swanson JW, DeRemee RA, Colby TV.
Neurologic manifestations of Churg-Strauss syndrome.
Mayo Clin Proc 1995;70:337-41. Crossref
6. Hattori N, Ichimura M, Nagamatsu M, et al.
Clinicopathological features of Churg-Strauss syndrome–associated neuropathy. Brain 1999;122:427-39. Crossref
7. Bosch X, Guilabert A, Espinosa G, Mirapeix E. Treatment
of antineutrophil cytoplasmic antibody associated
vasculitis: a systematic review. JAMA 2007;298:655-69. Crossref
8. Thiel J, Hässler F, Salzer U, Voll RE, Venhoff N. Rituximab
in the treatment of refractory or relapsing eosinophilic
granulomatosis with polyangiitis (Churg-Strauss
syndrome). Arthritis Res Ther 2013;15:R133. Crossref