Hong Kong Med J 2015 Dec;21(6):553–9 | Epub 6 Nov 2015
DOI: 10.12809/hkmj154557
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Use of insulin in diabetes: a century of treatment
Savita Shahani, MD1; Lokesh Shahani, MD2
1 Department of Pharmacology, MGM Medical College Mumbai (India), India
2 Department of Internal Medicine, Baylor College of Medicine, Houston, Texas, United States
Corresponding author: Dr Savita Shahani (drshahani@rediffmail.com)
Abstract
Insulin is a key player in the control of hyperglycaemia
for patients with type 1 diabetes mellitus and selected
patients with type 2 diabetes mellitus. There have
been many advances in insulin drug delivery from its
first administration as a crude pancreatic extract till
today. The traditional and most predictable method
for administration of insulin is by subcutaneous
injection. Currently available insulin delivery
systems include insulin syringes, infusion pumps,
jet injectors, and pens. The major drawback of
insulin therapy is its invasive nature. Non-invasive
delivery of insulin has long been a major goal for
the treatment of diabetes mellitus. Although there
have been improvements in insulin therapy since
it was first conceived, it is still far from mimicking
the physiological secretion of pancreatic β-cells,
and research to find new insulin formulations and
new routes of administration continues. This article
reviews the emerging technologies, including insulin
inhalers, insulin buccal spray, insulin pill, islet cell
transplant, and stem cell therapy, as treatment
options for diabetes mellitus.
Introduction
Diabetes mellitus is a major public health concern
worldwide. There is predicted to be an alarming
increase in the population with type 2 diabetes
mellitus both in developed and developing countries
over the next two decades. The prevalence of
diabetes among adults aged 20 to 70 years is
expected to rise from 285 million in 2010 to 438
million by the year 2030.1 Prevalences of diabetes
and impaired glucose tolerance are high in all Asian
countries and are expected to increase further in
the next 20 years. The present trend indicates that
more than 60% of the world’s diabetic population
will be in Asia.2 The prevalence of type 2 diabetes
is particularly high in Asian Indians because of high
genetic susceptibility and enhanced interaction with
environmental triggers. Exposure to a high fat diet
and low levels of physical activity are factors that can
trigger the gene-environment interaction.2 Therapy
with insulin is effective at lowering blood glucose in
patients with diabetes. Insulin is a key treatment in
the control of type 1 diabetes and it is required in the
later stages by patients with type 2 diabetes mellitus;
hyperglycaemia in type 1 diabetes is a result of
insulin deficiency and, in type 2 diabetes, it is due to
both impaired tissue response to insulin and insulin
deficiency. The discovery of insulin has been hailed
as one of the most dramatic events in the history of
the treatment of diabetes.
Before the discovery of insulin, diabetes
was a feared disease that led to death. Correlation
of destruction of the pancreas with diabetes was
observed in 1890 by von Mering and Minkowski,3
but internal secretion from the pancreas being
responsible for control of sugar was not identified.
In fact, the name ‘insulin’ was derived from the Latin
word ‘insula’ (meaning ‘island’) much earlier than
insulin was isolated.
As described by Bliss,4 between 1914 and 1916,
Paulesco and Zuelzer performed studies on dogs
with experimentally induced diabetes, showing the
antidiabetic effect of extract from the pancreas, but
they had to give up their experiments due to lack of
funds and the publication of their work was delayed
until July 1921. In 1922, Banting and Best5 confirmed
the antidiabetic effect of pancreatic extract in dogs
with experimentally induced diabetes. To carry on
with the experiments in a larger number of animals,
a substantial quantity of insulin was needed so
pancreatic extract from cows started being used.
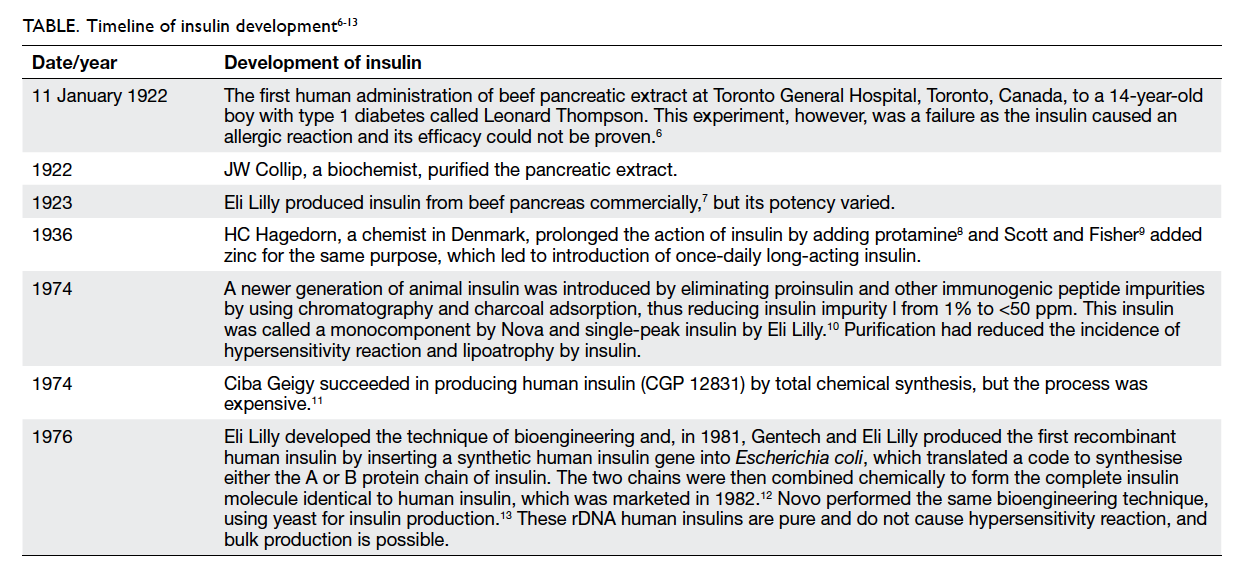
The timeline of insulin development is shown in the
Table.6 7 8 9 10 11 12 13
Alternative devices
At this stage (1984), insulin was only available in
vials and needed to be administered subcutaneously
via a syringe and needle, making it possible to mix
different types of insulin preparations. Patients,
however, found it inconvenient to administer
and there were mistakes in dosage measurement.
Therefore, alternative devices were developed to
improve drug delivery of insulin.
Insulin pen
The insulin pen combines an insulin container
and syringe in a single modular unit that makes it
convenient to administer insulin because of the ease
of insulin cartridge replacement. The insulin pen has
an inbuilt dial system that allows administration of
an accurate amount of drug. Pens are available as a
single premixed insulin administration unit.14
Insulin jet injector
The insulin jet injector has been designed to deliver
a fine spray of insulin subcutaneously at high speed
using a pressurised jet of air instead of a needle.
The dose is controlled by the dial-a-dose operation
through a single component design in comparison to
a conventional multi-component syringe. However,
the jet injector may cause bruising of the skin as
well as having altered absorption levels. The size
and cost of the jet injector further limit its routine
use. This device can be considered for patients with
needle phobia or those with severe insulin-induced
lipoatrophy.15
Injection port
The injection port is another device that functions as
a delivery channel directly into subcutaneous tissue.
The injection port contains an insertion needle
guiding a soft cannula into subcutaneous tissue.
Once applied, the insertion needle is removed and
a soft cannula remains in the subcutaneous tissue,
acting as gateway through which insulin can be
administered via a syringe or pen, thus avoiding
multiple pricks. The injection port can be changed
every third day. The device is available from
Medtronic (Dublin, Ireland).16
External insulin pump
The external insulin pump is a small battery-operated
device that is made up of an insulin reservoir
connected to a tube ending in a cannula that is
inserted under the skin of the abdomen and set to
deliver continuous subcutaneous insulin infusion
(CSII) throughout the day, and is programmed to
deliver insulin in a larger quantity at meal times.
This release pattern simulates physiological insulin
secretion, but bypasses the liver. Such CSII release
can be adjusted according to the specific needs of a
patient. Insulin delivery, however, may be interrupted
by infusion malfunction, needle displacement, pump
dysfunction, and lack of insulin in the reservoir,
therefore frequent blood glucose estimation is
required.17
Implantable insulin pump
An implantable insulin pump is a combination of a
continuous glucose sensor attached to a closed-loop
insulin infusion pump. The device is also known as an
artificial pancreas. Blood glucose control is achieved
by using wireless communication of a continuous
glucose monitor linked to an insulin infusion pump
that facilitates automated data transfer and delivers
insulin subcutaneously without the need for human
intervention.18 The lag period of human insulin
given subcutaneously by a pump is 60 to 90 minutes,
which can be minimised by using newer fast-acting
insulin analogues. Despite important developments
in sensor and pump technology, this device has
shown delays and inaccuracies in both glucose
sensing and insulin delivery, which is a problem
when controlling postprandial hyperglycaemia that
occurs substantially faster than the time needed for
insulin absorption and action. Suboptimal accuracy
and reliability remain one of the biggest obstacles for
closed-loop systems. Despite the substantial progress
made in recent years, there remains a number of
challenges to successful development of commercial
implantable insulin pump devices. These challenges
include the effect of exercise, concurrent illness,
large carbohydrate meals, and the pharmacokinetics
of current subcutaneous insulin.
Quick-acting insulin has a lag period of 60 to 90
minutes, therefore, it is required to be administered
30 minutes before meals to control postprandial
hyperglycaemia and may cause late postprandial
hypoglycaemia. To overcome this problem, rapid-acting
insulin analogues such as aspart, lispro, and
glulisine were synthesised by modifying amino
acid sequences in the insulin chain so as to keep
insulin in a monomeric form, which has a rapid
onset of action of 10 to 15 minutes, peak of 30 to
90 minutes, and duration of 3 to 4 hours resembling
physiological postprandial insulin secretion. Thus,
these analogues are very efficient at controlling
postprandial hyperglycaemia without the risk of
delayed hypoglycaemia.19 Research has been done
to enhance the onset of action of human insulin by
combining it with human hyaluronidase.20
One approach to creating ultrarapid-acting
insulin is use of a novel combination of excipients
to modify the insulin hexamer complex resulting
in more rapid dissociation of the hexamers into
monomers and dimers following subcutaneous
injection. Biodel Inc (Danbury [CT], US) has
developed a technology that facilitates more
rapid absorption of recombinant human insulin
than that of current insulin products. Biodel is
developing Linjeta (previously known as VIAject),
an ultrarapid-acting, injectable recombinant human
insulin formulation, which is currently under review
by the US Food and Drug Administration (FDA) to
compare its pharmacokinetic and pharmacodynamic
characteristics with rapidly acting insulin
analogues.21 BIOD-531 (Biodel Inc) has a more rapid
onset and longer duration of action than combined
prandial/basal insulins. The preclinical and
clinical data demonstrate its unique and attractive
pharmacokinetic and pharmacodynamic profiles.22
This formulation of recombinant human insulin has
the potential to provide improved glucose control
compared with insulin products designed to provide
both prandial and basal coverage in a single injection.
As BIOD-531 is a concentrated formulation (400
units/mL), the degree of glucose control can be
achieved with a small volume of injection.
Long-acting insulin analogues were designed
to obtain a steady basal insulin level compared
with older intermediate-acting insulin, which
has a risk of late-night hypoglycaemia. Insulin
glargine and insulin detemir were designed by
altering amino acid sequences in the human insulin
chain to make a slow-release preparation when
administered subcutaneously. Insulin degludec
is another long-acting insulin approved by the
US FDA in 2012 for basal blood sugar control. All
these long-acting peakless insulins are given once a
day to control basal blood sugar level without risk
of producing hypoglycaemia.23 PEGylated insulin
lispro (LY2605541) is a long-acting insulin whereby
the insulin molecule is embedded in a polyethylene
glycol (PEG) chain to increase the molecular size of
the insulin, thus reducing its rate of absorption.24
LY2605541 has completed phase 2 clinical trials.
Newer trends in needle-free insulin delivery systems
None of the above formulations delivered insulin
by needle-free technique, thus there is a continuing
search for a novel insulin delivery to overcome the
problem of needle prick. The newer trends for a
needle-free insulin delivery system are discussed as
follows.
Insulin inhaler
Owing to the large surface area, the lung is an
ideal target for drug delivery, and inhaled insulin
represents one of the most promising alternatives
to injectable insulin. Insulin has been developed
in powdered form. Exubera (Pfizer Inc, New
York [NY], US) was the first inhalable insulin
utilising recombinant human insulin. Exubera was
approved by US FDA in 2006 for both type 1 and
type 2 diabetics. As the inhaler contains short-acting
insulin, it can only control postprandial
hyperglycaemia. The powdered form of insulin can
sometimes stick together making it difficult to inhale
and reducing accuracy of the dose. A drawback of
Exubera was that it was a bulky inhaler device and
insulin was available in a blister packet that had to
be loaded into the inhaler device for each dose.25
Since insulin is known to have growth-promoting
properties by acting on insulin-like growth factor
receptors, clinicians have been concerned about
the possibility of long-term effects of intra-alveolar
deposition of insulin, although safety data collected
by Pfizer did not show any significant increase in
the incidence of lung malignancy in clinical trials.26
A systematic review showed that Exubera was not
superior to short-acting insulin in other formats
and was not cost-effective, so Pfizer discontinued
production.27 MannKind Corporation (Valencia
[CA], US) received US FDA approval in June 2014
for its ultrarapid-acting inhalation human insulin
powder, Afrezza, which contains recombinant
human insulin using the technosphere concept and
is administered via MannKind’s next-generation
inhaler called Dreamboat. Technosphere technology
is based on the pH-induced intermolecular self-assembly
of a novel small-molecule excipient
(fumaryl diketopiperazine). The technosphere drug
delivery system creates insulin microparticles (2-3 µm), which form microspheres that are lyophilised
into dry powder for inhalation, and dissolve
immediately once they come in contact with
alveoli. The peak plasma concentration is achieved
at 12 to 15 minutes,28 resembling physiological
postprandial insulin release, and thus it is required
to be administered just before meals, and controls
postprandial hyperglycaemia only. Afrezza was
approved by the US FDA for both type 1 and type
2 diabetes, with a label restriction for patients with
asthma, chronic obstructive pulmonary disease, or
lung cancer.
Mouth spray and adhesives
The buccal route is another promising alternative
for insulin delivery as this area has an abundant
blood supply, thus offering the possibility of delivery
of acid-labile insulin without undergoing first-pass
metabolism. Spray insulin preparations deliver
insulin in aerosol form, which is absorbed through
the inside of the cheek and the back of the mouth.
Generex Biotechnology Corporation (Toronto,
Canada) developed the buccal insulin formulation
Oral-lyn, which is a liquid formulation of regular
human insulin with a spray propellant using rapid
mist technology. Oral-lyn has an onset time of 5
minutes, with a peak of 30 minutes and duration
of 2 hours, and can be used to control postprandial
hyperglycaemia, having only 10% drug absorption.29
This formulation releases large micelles with a
particle size of >10 µm, so insulin does not reach the
lungs. The US FDA approved Oral-lyn for type 1 and
type 2 diabetes in 2009.
MidaSol Therapeutics (Oxford, UK), a joint
venture between nanotechnology firm Midatech
Pharma (Abingdon, UK) and drug delivery specialist
MonoSol Rx (Warren [NJ], US), has cleared its first
clinical hurdle in its bid to develop a gold-based
nanoparticle formulation of insulin that offers a
novel delivery route. A phase 1 trial of MidaForm
insulin, which is administered in a soluble strip that
adheres to the inside of the mouth, demonstrated
good safety and tolerability. The strip creates an
osmotic gradient across the buccal mucosa resulting
in rapid systemic delivery of insulin, with a peak
plasma concentration time of 5 to 8 minutes.30
Transdermal insulin
Transdermal insulin delivery could provide diabetic
patients with a sustained physiological level of basal
insulin in a pain-free manner. Dermal permeation
is limited to small lipophilic molecules, as the
stratum corneum is the major barrier to penetration.
Several physical enhancement techniques such
as iontophoresis, ultrasound, microneedles,
electroporation, lesser ablation, and chemical
enhancement have been explored to increase the
permeability of transdermal drugs.31 The U-Strip
(Ultrasonic Strip) Insulin Patch (Transdermal
Specialties, Inc, Broomall [PA], US) was designed
to deliver insulin lispro through the dermis using
alternating sonic waveforms to enlarge the diameter
of skin pores, enabling large-diameter molecules
to penetrate the stratum corneum.32 The U-Strip is
at an advanced stage of clinical trial, but is not yet
approved by the US FDA.
Oral insulin
The oral route of administration is considered to
be most acceptable and convenient for treatment
of chronic diseases. The concept of oral insulin
delivery has always been a challenge as the insulin
has to be protected from the acidic environment
of stomach and various metabolising enzymes, and
has poor permeability due to its hydrophilicity.
Oral insulin delivery has the advantage that it
delivers the drug through the portal circulation,
thus distributing a high concentration in the liver
resembling physiological insulin secretion. Various
attempts have been made to overcome the obstacles
of oral insulin therapy. Insulin has been complexed
with cyclodextrins in order to improve its solubility
and stability in the form of dry powder, after
encapsulation into poly(D,L-lactic-co-glycolic acid)
microspheres. Other attempts at oral insulin delivery
include incorporating insulin with a delivery agent
(sodium N-[8-(2-hydroxybenzoyl)amino] caprylate),
preparation of hyaluronan-insulin complex and
calcium phosphate-PEG-insulin-casein particles
for oral delivery.33 The physiological barriers to
absorption of oral insulin are its low bioavailability
and high inter-patient variability.
Biocon Ltd (Bangalore, India) has entered into
a research collaboration with Nobex Corporation
(Research Triangle Park [NC], US) to jointly develop
the oral insulin analogue IN-105. The recombinant
human insulin molecule has been modified by
linking a single short-chain amphiphilic oligomer,
through a covalent non-hydrolysable amide bond, to
the free amino acid group on the Lys-β29 residue.
This improved the solubility, stability, and systemic
absorption.34 From the results published so far,35 it
appears that IN-105 is a rapid-acting oral insulin
that could potentially have a place in the control of
postprandial hyperglycaemia. Biocon is collaborating
with Bristol-Myers Squibb (New York [NY], US) for
global clinical trials. In India, IN-105 has completed
a phase 2 clinical trial with promising results.36
Oramed Pharmaceuticals (Jerusalem, Israel)
was granted patent approval for its oral insulin
technology by the Japan Patent Office in April
2013. The POD (Protein Oral Delivery) technology
combines adjuvants that are capable of significantly
enhancing the absorption of peptides and proteins
across the intestinal wall when delivered orally
without modifying the active compounds. These
adjuvants are intended to protect the active peptide
or protein while in transit through the harsh
chemical environment of the gastro-intestinal tract
and promote its transport across the intestinal wall
into the general blood circulation. The tablet is
enteric-coated, thus preventing insulin release in
the stomach, and the increased pH of the intestines
signals it to open and the technology to start
functioning.37 The technology is in phase 2 clinical
trial, and preliminary data in patients with type 1
and type 2 diabetes are encouraging.38
Another oral insulin analogue under
development by Novo Nordisk (Bagsværd, Denmark)
incorporates Merrion Pharmaceuticals’ (Wilmington
[NC], US) Gastrointestinal Permeation Enhancement
Technology. This technology uses specially designed
oral formulations of absorption enhancers that
activate micelle formation, facilitating transport of
drug and substantially increasing absorption, with
good reproducibility. The specially coated tablets
are targeted to dissolve in the duodenum releasing
both drug and absorption enhancer, which may pass
through the duodenal cell membrane. Novo Nordisk
has completed a phase 1 study of oral insulin NN1952
as well as another molecule, NNC0148-0000-0362
(NN1954), in healthy participants and patients with
type 1 and type 2 diabetes.39
There is renewed hope for the treatment of
type 1 diabetes with gel capsules. The Norwegian
University of Science and Technology has developed
a new type of capsule called Trondheim Alginate
Microcapsule, which is designed to camouflage the
insulin-producing cells from the body’s immune
system. If this becomes a medical reality, diabetic
patients with transplanted insulin-producing donor
cells in their abdominal cavity might not have to take
immunosuppressants for the rest of their lives.40
Pancreas transplantation and stem cell therapy
Intensified exogenous insulin therapy rarely
attains normal blood glucose levels without risk
of major hypoglycaemic episodes, and cannot
approximate normal physiological pulsatile
insulin secretary patterns with complete integrity.
Pancreas transplantation is the only therapy shown
to stop the progression of diabetic complications
without increasing the incidence of hypoglycaemic
events. Whole pancreas transplantation was first
performed for treatment of diabetes in 1966.41
While percutaneous islet cell transplantation is a
minimally invasive cellular replacement therapy that
was developed to avoid the surgical complications of
whole pancreas transplantation,42 both procedures
require immunosuppressant therapy.
Developments in this fast-moving area of
research have focused on the principle of generating
insulin-expressing cells from stem cells, with the
possibility of generating unlimited numbers of
functional beta cells for transplantation therapy. The
adoption of a stem cell–based therapy for diabetes,
however, will depend on it being shown to be as safe
and effective as the current therapy of administration
of exogenous insulin.
The stem cell populations that have been used
in experimental studies can be tissue stem cells,
defined as multipotent progenitor cells found in fetal
and adult tissues; embryonic stem cells, defined as
pluripotent, undifferentiated cells generated from the
inner cell mass of a developing blastocyst; or induced
pluripotent stem cells, defined as pluripotent cells
generated by reprogramming differentiated adult
cells by forced expression of pluripotency genes.
The pluripotency and proliferative potential of stem
cell populations raises the undesirable possibility of
uncontrolled cellular proliferation and formation
of teratomas after transplantation, which has been
reported in animal studies.43 Autologous grafting
of insulin-secreting cells derived from the patient’s
own tissue stem cells is attractive, but experimental
studies have not yet translated into clinically useful
material, mainly because of problems with restricted
proliferative capacity, causing low levels of insulin.
Thus, in spite of the impressive promise of stem cells,
no proven benefits have been demonstrated by stem
cell therapy in the treatment of diabetes.
Xenotransplantation refers to the
transplantation of tissue or organs from one
species to another. This therapy offers the ability to
overcome the problem of transplant organ shortage.
At present, pigs are thought to be the best candidates
for xenotransplant donation, as they are plentiful,
quick to mature, and breed well. Transplantation of
porcine islet cells into non-human primates has been
successfully performed with encouraging results,
including longer graft survival,44 but the benefits
of xenotransplantation must be weighed against
the potential for interspecies transmission of viral
infection and issues related to incongruity of tissue
ageing between humans and swine.
Conclusion
Insulin has saved the lives of countless people
since its discovery as a pancreatic crude extract.
The advances made in insulin delivery could surely
provide intensive insulin therapy regimens that can
reduce the multiple daily subcutaneous injections
and heavy burden of compliance on patients. Research
and development in insulin delivery technology
has opened new avenues that can be explored for
the cure and control of insulin-dependent diabetes
mellitus. The day does not seem to be far away when
the parenteral route of insulin administration, which
has been the only suitable route, will be outdated and
patients will be using alternative routes with ease and
comfort. Alternative technologies for the delivery of
insulin will be a major breakthrough in changing the
lifestyles of millions of diabetic patients around the
globe. Therefore, research and investigation into the
development of safer and more effective systems for
delivery of insulin must continue.
Declaration
No conflicts of interests were declared by authors.
References
1. Unwin N, Whiting D, Gan D, Jacqmain O, Ghyoot G,
editors. IDF Diabetes Atlas. 4th ed. Brussels: International
Diabetes Federation; 2009.
2. Ramachandran A, Snehalatha C, Shetty AS, Nanditha A.
Trends in prevalence of diabetes in Asian countries. World
J Diabetes 2012;3:110-7. Crossref
3. von Mering J, Minkowski O. Diabetes mellitus nach
pankreas extirpation. Arch Exp Pathol Pharmacol
1890;26:371-87. Crossref
4. Bliss M. The history of insulin. Diabetes Care 1993;16
Suppl 3:4-7. Crossref
5. Banting FG, Best CH. Internal secretion of the pancreas. J
Lab Clin Med 1922;7:251-66.
6. Banting FG. The history of insulin. Edinburg Med J
1929;36:1-18.
7. Discovery of insulin: A medical marvel for the sugar
sickness. Available from: http://www.trumanlibrary.org/histday/insulin/eli-lilly-and-company.html. Accessed Mar
2015.
8. Hagedorn HC, Jensen BN, Krarup NB, Wodstrup I.
Protamine insulinate. JAMA 1936;106:177-80. Crossref
9. Scott DA, Fisher AM. The effect of zinc salts on the action
of insulin. J Pharmacol Exp Ther 1935;55:206-21.
10. Deckert T, Andersen OO, Poulsen JE. The clinical
significance of highly purified pig-insulin preparations.
Diabetologia 1974;10:703-8. Crossref
11. Teuscher A. The biological effect of purely synthetic
human insulin in patients with diabetes mellitus. Schweiz
Med Wochenschr 1979;109:743-7.
12. Keen H, Glynne A, Pickup JC, et al. Human insulin
produced by recombinant DNA technology: safety and
hypoglycaemic potency in healthy men. Lancet 1980;2:398-401. Crossref
13. Markussen J, Damgaard U, Pingel M, Snel L, Sørensen
AR, Sørensen E. Human insulin (Novo) chemistry and
characteristics. Diabetes Care 1983;6 Suppl 1:4-8.
14. Magwire ML. Addressing barriers to insulin therapy: the
role of insulin pens. Am J Ther 2011;18:392-402. Crossref
15. Logwin S, Conget I, Jansa M, Vidal M, Nicolau C, Gomis R.
Human insulin-induced lipoatrophy. Successful treatment
using a jet-injection device. Diabetes Care 1996;19:255-6. Crossref
16. Blevins T, Schwartz SL, Bode B, et al. A study assessing
an injection port for administration of insulin. Diabetes
Spectr 2008;21:197-201. Crossref
17. Dandona P, Foster M, Healey F, Greenbury E, Beckett AG.
Low-dose insulin infusions in diabetic patients with high
insulin requirements. Lancet 1978;2:283-5. Crossref
18. Peyser T, Dassau E, Breton M, Skyler JS. The artificial
pancreas: current status and future prospects in the
management of diabetes. Ann N Y Acad Sci 2014;1311:102-23. Crossref
19. Powers AC, D’Alessio D. Endocrine pancreas and
pharmacotherapy of diabetes mellitus and hypoglycemia.
In: Goodman and Gilman’s: The pharmacological basis of
therapeutics. 12th ed. New Delhi: McGraw Hill; 2011: 1237-73.
20. Hompesch M, Muchmore DB, Morrow L, Vaughn DE.
Accelerated insulin pharmacokinetics and improved
postprandial glycemic control in patients with type 1
diabetes after coadministration of prandial insulins with
hyaluronidase. Diabetes Care 2011;34:666-8. Crossref
21. Steiner S, Hompesch M, Pohl R, et al. A novel insulin
formulation with a more rapid onset of action. Diabetologia
2008;51:1602-6. Crossref
22. Morrow L, Hompesch M, Canney L, Pichotta P,
Krasner A, de Souza E. Biphasic pharmacokinetic and
pharmacodynamic profiles associated with concentrated
insulin BIOD-531 show rapid onset and basal duration of
action. Poster presented at the EASD Annual Meeting 2014;
15-19 September 2014; Vienna, Austria. Abstract #937.
23. Tahrani AA, Bailey CJ, Barnett AH. Insulin degludec: a
new ultra-longacting insulin. Lancet 2012;379:1465-7. Crossref
24. Rosenstock J, Bergenstal RM, Blevins TC, et al. Better
glycemic control and weight loss with the novel long-acting
basal insulin LY2605541 compared with insulin glargine in
type 1 diabetes: a randomized, crossover study. Diabetes
Care 2013;36:522-8. Crossref
25. Barnett AH. Exubera inhaled insulin: a review. Int J Clin
Pract 2004;58:394-401. Crossref
26. Barnett AH, Lange P, Dreyer M, Serdarevic-Pehar M;
Exubera Phase 3 Study Group. Long-term tolerability of
inhaled human insulin (Exubera) in patients with poorly
controlled type 2 diabetes. Int J Clin Pract 2007;61:1614-25. Crossref
27. Black C, Cummins E, Royle P, Philip S, Waugh N. The
clinical effectiveness and cost-effectiveness of inhaled
insulin in diabetes mellitus: a systematic review and
economic evaluation. Health Technol Assess 2007;11:1-126. Crossref
28. Rosenstock J, Lorber DL, Gnudi L, et al. Prandial inhaled
insulin plus basal insulin glargine versus twice daily biaspart
insulin for type 2 diabetes: a multicentre randomised trial.
Lancet 2010;375:2244-53. Crossref
29. Modi P, Mihic M, Lewin A. The evolving role of oral insulin in
the treatment of diabetes using a novel RapidMist System.
Diabetes Metab Res Rev 2002;18 Suppl 1:S38-42. Crossref
30. Soares S, Costa A, Sarmento B. Novel non-invasive
methods of insulin delivery. Expert Opin Drug Deliv
2012;9:1539-58. Crossref
31. Park EJ, Dodds J, Smith NB. Dose comparison of ultrasonic
transdermal insulin delivery to subcutaneous insulin
injection. Int J Nanomedicine 2008;3:335-41.
32. Insulin patch offers hope of needle free diabetes
management. Unveiled at American Diabetes
Association’s 72nd Annual Scientific Meeting on June 9-11
2012 in Philadelphia. Heritage. Eli Lilly and Company.
Available from: http://srxawordonhealth.com/2012/06/01/insulin-patch-offers-hope-of-needle-free-diabetes-management/.
Accessed Sep 2015.
33. Semalty A, Semalty M, Singh R, Saraf SK, Saraf S.
Properties and formulation of oral drug delivery systems of
protein and peptides. Indian J Pharm Sci 2007;69:741-7. Crossref
34. Kinesh VP, Neelam DP, Punit BP, Bhavesh SB, Pranga
KS. Novel approach for oral delivery of insulin and
current status of oral insulin products. Int J Pharm Sci
Nanotechnology 2010;3:1057-64.
35. Heinemann L, Jacques Y. Oral insulin and buccal insulin: a
critical reappraisal. J Diabetes Sci Technol 2009;3:568-84. Crossref
36. Khedkar A, Iyer H, Anand A, et al. A dose range finding
study of novel oral insulin (IN-105) under fed conditions
in type 2 diabetes mellitus subjects. Diabetes Obes Metab
2010;12:659-64. Crossref
37. Kidron M, Arbit E, Shushlav Y. Comparative assessment
of the glucose-lowering effect of multiple oral insulin
(ORMD-0801) formulation variant in pigs. Paper presented
at the 74th Scientific Sessions of the American Diabetes
Association; 13-17 June 2014; San Francisco, California,
USA.
38. Neutel J, Kidron M, Arbit E, Homer K. Bedtime oral insulin
lowers fasting blood glucose levels in T2DM patients.
Poster presented at the 74th Scientific Sessions of the
American Diabetes Association; 13-17 June 2014; San
Francisco, California, USA.
39. A trial investigating the safety, tolerability, pharmacokinetics
and pharmacodynamics of NNC0148-0000-0362 in
healthy subjects. Available from: https://clinicaltrials.gov/ct2/show/NCT01597713. Accessed Sep 2015.
40. de Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in
cell encapsulation from an enveloped cell perspective. Adv
Drug Deliv Rev 2014;67-68:15-34. Crossref
41. Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC.
Allotransplantation of the pancreas and duodenum
along with the kidney in diabetic nephropathy. Surgery
1967;61:827-37.
42. Hatipoglu B, Benedetti E, Oberholzer J. Islet
transplantation: current status and future directions. Curr
Diab Rep 2005;5:311-6. Crossref
43. Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted
human and rat beta cells. Diabetologia 2008;51:91-100. Crossref
44. Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged
diabetes reversal after intraportal xenotransplantation of
wild-type porcine islets in immunosuppressed nonhuman
primates. Nat Med 2006;12:301-3. Crossref