Hong Kong Med J 2015 Oct;21(5):411–6 | Epub 28 Aug 2015
DOI: 10.12809/hkmj154542
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Corticosteroid adulteration in proprietary Chinese medicines: a recurring problem
YK Chong, MB, BS;
CK Ching, FRCPA, FHKAM (Pathology);
SW Ng, MPhil;
Tony WL Mak, FRCPath, FHKAM (Pathology)
Hospital Authority Toxicology Reference Laboratory, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr Tony WL Mak (makwl@ha.org.hk)
Abstract
Objectives: To investigate adulteration of
proprietary Chinese medicines with corticosteroids
in Hong Kong.
Design: Case series with cross-sectional analysis.
Setting: A tertiary clinical toxicology laboratory in
Hong Kong.
Patients: All patients using proprietary Chinese
medicines adulterated with corticosteroids and
referred to the authors’ centre from 1 January 2008
to 31 December 2012.
Main outcome measures: Patients’ demographic
data, clinical presentation, medical history, drug
history, laboratory investigations, and analytical
findings of the proprietary Chinese medicines were
analysed.
Results: The records of 61 patients who consumed
corticosteroid-adulterated proprietary Chinese
medicines were reviewed. The most common
corticosteroid implicated was dexamethasone. Co-adulterants
such as non-steroidal anti-inflammatory
drugs and histamine H1-receptor antagonists were
detected in the proprietary Chinese medicine
specimens. Among the patients, seven (11.5%)
required intensive care, two (3.3%) died within 30
days of presentation, and 38 (62.3%) had one or more
complications that were potentially attributable to
exogenous corticosteroids. Of 22 (36.1%) patients
who had provocative adrenal function testing
performed, 17 (77.3% of those tested) had adrenal
insufficiency.
Conclusion: The present case series is the largest
series of patients taking proprietary Chinese
medicines adulterated with corticosteroids.
Patients taking these illicit products are at risk of
severe adverse effects, including potentially fatal
complications. Adrenal insufficiency was very
common in this series of patients. Assessment
of adrenal function in these patients, however,
has been inadequate and routine rather than
discretionary testing of adrenal function is indicated
in this group of patients. The continuing emergence
of proprietary Chinese medicines adulterated with
western medication indicates a persistent threat to
public health.
New knowledge added by this
study
- Adulteration of proprietary Chinese medicines (pCMs) with corticosteroids is a significant yet underrecognised phenomenon. Co-adulteration with non-steroidal anti-inflammatory drugs and histamine H1-receptor antagonists is often seen.
- Adrenal insufficiency is a common complication in patients who have consumed pCMs adulterated with corticosteroids.
- Adrenal function testing is essential for patients suspected to have taken corticosteroid-adulterated pCMs.
- Public health education on the danger of taking pCMs of dubious sources and implementation of effective regulatory measures are important to address the problem of corticosteroid-adulterated pCMs.
Introduction
Proprietary Chinese medicines (pCMs) are products
claimed to be made of Chinese medicines and
formulated in a finished dosage form. As with
traditional Chinese medicine, pCMs are generally
regarded by the public as benign and non-toxic, as
compared with western medications.
Undeclared corticosteroids, among other
adulterants, have been reported to be added to
pCMs, Ayurvedic medicine, and homeopathic
medicine.1 2 3 4 5 There are multiple incentives for adding
corticosteroids: they have powerful analgesic and
anti-inflammatory actions, making these proprietary
products effective against various allergic,
autoimmune, dermatological, and musculoskeletal
pain disorders.
From 2008 to 2012, the Hospital Authority
Toxicology Reference Laboratory, the only tertiary
referral centre for clinical toxicological analysis in
Hong Kong, confirmed 61 cases of corticosteroid-adulterated
pCMs. We report these cases to highlight
the severity and danger of using such adulterated
medications.
Methods
From 1 January 2008 to 31 December 2012, all
cases referred to the Hospital Authority Toxicology
Reference Laboratory that involved the use of
pCMs, which were subsequently found to contain
corticosteroids, were retrospectively reviewed.
Clinical data were obtained by reviewing the
laboratory database as well as the patients’ electronic
and, where necessary, paper health records.
Demographic data, clinical presentation, medical
history, drug history, laboratory investigations,
radiological investigations, and analytical findings
of the pCMs were reviewed. For the evaluation of
adrenocortical function, due to the heterogeneity
of patient population and the nature of the
retrospective case series for the present study, both
low-dose short synacthen test (LDSST) and short
synacthen test (SST) have been used for the diagnosis
of adrenal insufficiency. We adopted a cutoff of
550 nmol/L, which has been traditionally used for
SST, and previously validated in the local population
for LDSST.6
The presumed causal relationship between
the clinical features or complications or both of the
patients and the adulterants was evaluated based on
the temporal sequence, the known adverse effects of
the detected drugs, and the presence of underlying
diseases.
The presence of corticosteroids was analysed
qualitatively by liquid chromatography–tandem
mass spectrometry (LC-MS/MS) with a linear
ion trap instrument. The presence of other co-adulterants
was analysed qualitatively by high-performance
liquid chromatography with diode-array
detection. Confirmations by LC-MS/MS or
gas chromatography–mass spectrometry were
performed as required.
This study was approved by the Hong Kong
Hospital Authority Kowloon West Cluster Research
Ethics Committee (approval number KW/EX-13-121). The Committee exempted the study group from
obtaining patient consent because the presented
data were anonymised, and the risk of identification was
low.
Results
A total of 61 patients involving the use of 61
corticosteroid-adulterated pCMs were referred
to the Hospital Authority Toxicology Reference
Laboratory in Hong Kong. There were 30 men and 31
women, with an age range of 1 to 91 years (median,
65 years). Seven (11.5%) patients were younger than
18 years. The usage duration ranged from 3 days to
10 years, with a median of 4 months.
Most (n=47, 77.0%) patients obtained the corticosteroid-adulterated
pCMs over-the-counter and 13 (21.3%) obtained the
steroid-adulterated pCMs from Chinese medicine
practitioners. The source for one case remained
unknown. Among the 47 patients who obtained their
pCMs over-the-counter, 38 (80.9%) obtained the pCMs in the Mainland China, seven
(14.9%) obtained the pCMs in Hong Kong,
and the remaining two patients (each accounting for
2.1%) obtained the pCMs from Taiwan and Malaysia.
For patients who obtained their pCMs from
Chinese medicine practitioners (n=13), the practitioners
were located in Hong Kong in nine (69.2%),
Mainland China in two (15.4%), and Macau in
two (15.4%) cases.
The three most common indications for the
use of pCMs were musculoskeletal pain (n=36;
59.0%), skin disorders such as eczema and psoriasis
(n=13; 21.3%), and airway problems such as asthma,
bronchiectasis, and chronic obstructive airway
disease (n=8; 13.1%). The indications for all seven
(11.5%) paediatric patients were for skin disorders.
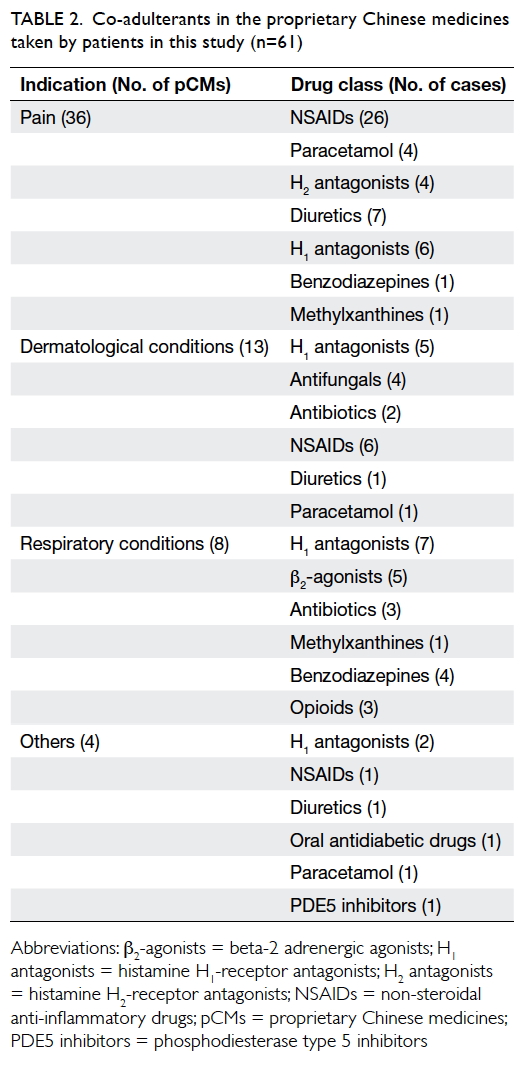
Dexamethasone, present in 29 (47.5%) pCMs,
and prednisone, present in 21 (34.4%) pCMs, were
the most common corticosteroid adulterants among
the pCMs submitted for analysis. Details of the
corticosteroid adulteration are listed in Table 1.
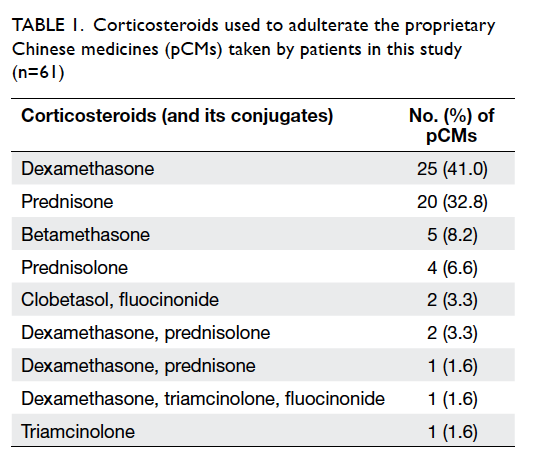
Table 1. Corticosteroids used to adulterate the proprietary Chinese medicines (pCMs) taken by patients in this study (n=61)
Other than steroids, co-adulterants were also
detected in 53 (86.9%) pCMs. The most common co-adulterants
were non-steroidal anti-inflammatory
drugs (NSAIDs; present in 33 [54.1%] pCMs) and
histamine H1-receptor antagonists (present in 20
[32.8%] pCMs). The co-adulterants are listed in Table 2.
Overall, 38 (62.3%) patients had one or more
complications that were either attributable or
potentially attributable to the use of exogenous
corticosteroids: 18 (29.5%) were documented to
have clinical Cushing’s syndrome; eight (13.1%) had
endoscopic-proven gastritis or peptic ulcer disease,
of whom six (9.8%) were proven to be Helicobacter
pylori–negative; five (8.2%) had sepsis at presentation;
three (4.9%) had hepatitis B exacerbation; and two
(3.3%) had tuberculosis. Other clinical presentations
included hepatitis C reactivation, transient diabetes
that resolved after discontinuation of corticosteroids,
and cataract occurring in a paediatric patient.
Overall, 22 (36.1%) patients had adrenal function
testing performed, and among them 17 (77.3%) had
biochemically confirmed adrenal insufficiency.
For the subgroup in whom Cushing’s
syndrome was not identified (n=43; 70.5%), LDSST/SST were performed in 11 (25.6%), and among those,
seven (63.6%) had biochemically confirmed adrenal
insufficiency.
Seven (11.5%) patients in this series required
intensive care, and two (3.3%) died within 1 month
of initial presentation. Among the patients who had
consumed pCMs adulterated with corticosteroids
and required intensive care unit admission, the
clinical presentations of two patients may have been
related to the use of corticosteroids, which are described below.
Case 1
The patient was a 67-year-old man who had a
history of psoriasis, diabetes, hypertension, and
chronic renal impairment. He presented in 2012
with fever, decreased urine output, and gastro-intestinal
upset. He reported a 2-month history of
using a pCM for psoriasis, and his skin condition
dramatically improved. He was in shock on
admission, with acute renal failure and respiratory
distress. He was admitted to the intensive care unit
where he stayed for 7 days. He required inotropic
support and mechanical ventilation. Computed
tomography revealed a large lung abscess and blood
culture showed Pseudomonas species. During his
hospitalisation, SST was performed, and the results
were adequate (cortisol level of 944 nmol/L at 30 minutes
after synacthen injection).
In the pCM submitted for analysis, prednisone
acetate was detected, among other herbal markers.
His condition improved with drainage of the abscess
and prolonged intravenous antibiotics, including
cefoperazone and sulbactam (1 g and later 2 g every
12 hours intravenously [IV] for 39 days) as well as
imipenem and cilastatin (500 mg every 8 hours
IV for 51 days). He was discharged after a long
rehabilitation programme, 3 months after the initial
admission.
Case 2
The patient was a 61-year-old man. He presented
in 2009 with a history of asthma, and was a chronic
smoker. He initially presented with low back pain
after slipping and falling. He, however, was noted to
have bilateral apical opacities on chest radiograph,
and was found to have smear-positive, open
pulmonary tuberculosis.
He was put on piperacillin (4 g every 6
hours IV), augmentin (1.2 g every 8 hours IV),
clarithromycin (500 mg twice a day orally), isoniazid
(300 mg daily orally), rifampicin (450 mg daily
orally), and ethambutol (700 mg daily orally) initially
while he was intubated, ventilated, and admitted to
intensive care unit for respiratory failure. During
his initial improvement in the intensive care unit,
he reported the use of a kind of herbal powder,
which he took to alleviate his airway condition. In
the herbal powder, opium alkaloids (morphine,
codeine), oxytetracycline, diazepam, clenbuterol,
and prednisone were detected, among other herbal
markers.
His condition later deteriorated and he went
into respiratory failure and required intubation.
Subsequently, he died of ventilator-associated
Escherichia coli pneumonia. In this patient,
adrenal function testing with LDSST/SST was not
performed.
Discussion
Corticosteroids are notorious for causing side-effects
such as Cushing’s syndrome, adrenal insufficiency,
cataracts, peptic ulcer disease, osteoporosis, and
decreased immune response, particularly when used
for a protracted period of time in high doses. The
latest Endocrine Society guidelines on the diagnosis
of Cushing’s syndrome has also stressed obtaining
a thorough history to exclude excessive exogenous
glucocorticoid exposure leading to iatrogenic
Cushing’s syndrome.7 The continuing emergence
of corticosteroid-adulterated pCMs indicates that
iatrogenic Cushing’s syndrome is a persistent
problem with public health implications.
The incentive behind adulteration of pCMs is
easily understandable. Most of the pCMs involved
suggest that they are useful for the treatment of pain,
skin problems, or respiratory ailments. Steroids,
notwithstanding their many adverse effects, are
effective therapy for pain, inflammatory disorders,
allergic skin problems, and respiratory disorders
such as asthma and chronic obstructive airway
disease.
Although the side-effects of corticosteroids
have been extensively described over the past century,
many of these effects are multifactorial in their
pathophysiology, and the effect of corticosteroids
is difficult to quantitate in isolation. For example,
Cushing’s syndrome and adrenal insufficiency as
adverse drug reactions associated with the use of
corticosteroid-adulterated pCMs are less likely to
be disputed, for example H pylori–negative peptic
ulcer disease can be due to stress, alcoholism, use of
NSAIDs, and other concomitant illnesses.
Despite the presence of confounding factors,
the adverse effects of corticosteroid use are suspected
in many of the patients who use corticosteroid-containing
pCMs: for example, the deep-seated
infection in patient 1 and open tuberculosis in patient
2 could well be a result of immunosuppression due to
the use of corticosteroids. For the paediatric patient
with cataract on presentation, given that the patient
had no other clinical features to suggest a metabolic
or exogenous cause for the cataract, it is more likely
that the presence of the cataract was due to the
use of corticosteroids. The authors considered all
cases of Cushing’s syndrome, adrenal insufficiency,
and cataract occurring in paediatric patients to be
attributable to the use of exogenous steroids. The
prevalence of these conditions in this case series and
other conditions that are potentially attributable to
the use of exogenous corticosteroids are listed in
Table 3.
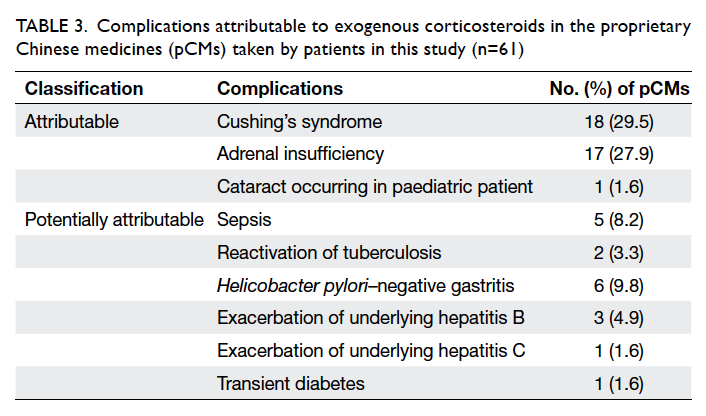
Table 3. Complications attributable to exogenous corticosteroids in the proprietary Chinese medicines (pCMs) taken by patients in this study (n=61)
The presence of co-adulterants in steroid-adulterated
pCMs appears to be the rule rather than
the exception. It cannot be overstressed that co-adulterants
present in pCMs are equally dangerous,
even when compared with corticosteroids, for
example, the presence of multiple NSAIDs
together with steroids puts patients at high risk
for complications (such as acute kidney injury and
peptic ulcer disease), and opiates (such as codeine
and morphine) present in pCMs indicated for
respiratory conditions puts patients, who most likely
have asthma or chronic obstructive airway disease,
at high risk for respiratory depression and carbon
dioxide narcosis. While effective at ameliorating
symptoms, these drugs delay the clinical presentation
and hence the opportunity to treat the disease at an
early stage.
Many therapeutically irrelevant medications
were also found in the pCMs. Examples include
histamine H1-receptor antagonists found in
adulterated pCMs that are intended to treat bone
pain, and the presence of tadalafil (a drug used to
treat erectile dysfunction) found in an adulterated
pCM that is supposed to treat diabetes.
It is difficult to comprehend the reason behind
the addition of such co-adulterants, although
contamination due to poor pharmaceutical
manufacturing practice is likely a contributing
factor, if not the sole reason.
For the diagnosis of exogenous corticosteroid
intake, maintaining a high index of suspicion is of
utmost importance. The classical feature of Cushing’s
syndrome was present in less than 30% of patients in
this series. This indicates that a large proportion of
cases would likely be missed if biochemical testing
was only performed following demonstration of
classical features of exogenous steroid intake. This
experience indicates that it is often worthwhile
testing patients who improve dramatically with the
use of pCMs from dubious sources, especially when
the treatment claims to be effective for treating
pain, airway diseases, and childhood eczema. In
these cases, a detailed drug history, and laboratory
analysis of suspicious pCMs can help to confirm the
diagnosis.
The management of these patients starts with
termination of exposure to the adulterated pCMs,
and treatment of the complications that have already
occurred. It is prudent to provide corticosteroid
replacement therapy pending dynamic function test
for adrenal function. For patients with underlying
inflammatory or autoimmune disorders such as
gouty arthritis, psoriasis, and eczema, abrupt
discontinuation of corticosteroid medications
may trigger an exacerbation of disease. In these
groups of patients, slow, gradual tapering should be
considered.
A worrying observation in the present series
is the occurrence of adrenal insufficiency, as well as
the lack of investigations thereof. Patients who were
exposed to pCMs adulterated with corticosteroids
were clearly at risk of adrenal insufficiency due
to suppression of adrenocorticotropic hormone
secretion and the resultant adrenocortical atrophy.
In this series, LDSST/SST was only performed
in 36.1% of the patients, and in those patients in whom
the tests were performed, 77.3% were inadequate.
It is clear that, among the patients who were not
tested, some were likely to have undiagnosed adrenal
insufficiency. As undiagnosed adrenal insufficiency
carries a high risk of morbidity and mortality, the
authors believe that LDSST should be performed on
all patients who have significant exposure to pCMs
adulterated with corticosteroids, even if they have
no signs of Cushing’s syndrome.
While spot cortisol obtained in the morning
is diagnostic if it is <100 nmol/L or >420 nmol/L
as verified locally by Choi et al,6 we recommend
LDSST as the test for adrenal function; LDSST
(1 µg) is preferred over the standard (250 µg) SST
because studies indicate that LDSST may be more
sensitive in detecting partial adrenal insufficiency.8 9
The authors further recommend that a sensitive
cutoff of 550 nmol/L at 30 minutes be used for the
purpose of diagnosing adrenal insufficiency in this
group of patients. Our recommendation for use of
provocative adrenal testing and a sensitive cutoff
level is based on the high probability of adrenal
insufficiency in this group of patients.
Prevention is always better than cure, and
this is especially true for public health issues. While
analytical and clinical toxicologists are well aware
of the situation, it is important to bring this matter
to the other stakeholders in society, namely, policy-makers, frontline clinicians, and the general public,
with communication tailored to the recipients.
For the general public, a simple rule can be
taught: if it sounds too good to be true, it probably
is; and this is especially so for pCMs that claim to
treat certain conditions in which western-drug
adulteration is common, for example, weight
reduction and diabetes, as previously reported by
our unit,10 11 and pain, respiratory conditions, and
skin problems, as reported in the present study. It
is prudent to consider brands and retailers that are
trustworthy and, in case of doubt, patients should
seek opinion from their primary care doctors.
For frontline clinicians, we wish to bring
to their attention that this adulteration issue is
common, recurring, and worthy of consideration,
and that patients who have a history of using such
corticosteroid-adulterated pCMs should have their
adrenal function tested. It is also important that
iatrogenic Cushing’s syndrome subsequent to the
use of corticosteroids that are from a source of
adulteration be reported to the relevant authorities.
It is the opinion of the authors that liberal, but careful,
reporting would contribute to better understanding
of this problem, further the prosecution of those
behind steroid-adulteration of pCMs, and help to
ameliorate this public health problem.
As for legislation and policies, consideration of
fraudulent prescription contrary to the expectation
of patients, who would expect traditional Chinese
medicine rather than the inappropriate use of
corticosteroids seen in many of these cases, by the
legislators, judiciary, and relevant councils and
constituents, rather than focusing on the possession
and unlawful sale of the relevant compounds, would
be a great deterrent to these illicit practices.
Conclusion
The present case series is the largest series of patients
using pCMs adulterated with corticosteroids. The
continuing emergence of pCMs adulterated with
western medications indicates a persistent threat to
public health. It is thus important that the risk be
communicated not only to the medical profession,
but also to the public, and effective regulatory
measures to combat these illicit pCMs should be in
place.
References
1. Ahmed S, Riaz M. Quantitation of cortico-steroids
as common adulterants in local drugs by HPLC. Chromatographia 1991;31:67-70. Crossref
2. Hon KL, Leung TF, Yau HC, Chan T. Paradoxical use of oral
and topical steroids in steroid-phobic patients resorting to
traditional Chinese medicines. World J Pediatr 2012;8:263-7. Crossref
3. Ku Y, Wen K, Ho L, Chang YS. Solid-phase extraction and
high performance liquid chromatographic determination
of steroids adulterated in traditional Chinese medicines. J
Food Drug Anal 1999;7:123-30.
4. Morice A. Adulteration of homoeopathic remedies. Lancet 1987;329:635. Crossref
5. Savaliya AA, Prasad B, Raijada DK, Singh S. Detection and
characterization of synthetic steroidal and non-steroidal
anti-inflammatory drugs in Indian ayurvedic/herbal
products using LC-MS/TOF. Drug Test Anal 2009;1:372-81. Crossref
6. Choi CH, Tiu SC, Shek CC, Choi KL, Chan FK, Kong PS.
Use of the low-dose corticotropin stimulation test for the
diagnosis of secondary adrenocortical insufficiency. Hong
Kong Med J 2002;8:427-34.
7. Nieman LK, Biller BM, Findling JW, et al. The diagnosis
of Cushing’s syndrome: an Endocrine Society Clinical
Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40. Crossref
8. Broide J, Soferman R, Kivity S, et al. Low-dose
adrenocorticotropin test reveals impaired adrenal function
in patients taking inhaled corticosteroids. J Clin Endocrinol
Metab 1995;80:1243-6. Crossref
9. Streeten DH, Anderson GH Jr, Bonaventura MM. The
potential for serious consequences from misinterpreting
normal responses to the rapid adrenocorticotropin test. J
Clin Endocrinol Metab 1996;81:285-90. Crossref
10. Ching CK, Lam YH, Chan AY, Mak TW. Adulteration
of herbal antidiabetic products with undeclared
pharmaceuticals: a case series in Hong Kong. Br J Clin
Pharmacol 2012;73:795-800. Crossref
11. Tang MH, Chen SP, Ng SW, Chan AY, Mak TW. Case
series on a diversity of illicit weight-reducing agents: from
the well known to the unexpected. Br J Clin Pharmacol
2011;71:250-3. Crossref