Hong Kong Med J 2015 Oct;21(5):401–6 | Epub 31 Jul 2015
DOI: 10.12809/hkmj144339
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Angiographic factors associated with haemorrhagic presentation of brain arteriovenous
malformation in a Chinese paediatric population
Elaine WS Fok, FRCR, FHKCR1;
WL Poon, FRCR, FHKAM (Radiology)1;
KS Tse, FRCR, FHKAM (Radiology)1;
HY Lau, FRCR, FHKAM (Radiology)1;
CH Chan, MB, BS, FRCR2;
NY Pan#, FRCR, FHKAM (Radiology)2;
HY Cho, FRCR, FHKAM (Radiology)2;
TW Yeung, FRCR, FHKCR3;
YC Wong, FRCR, FHKAM (Radiology)3;
KW Leung, FRCR, FHKAM (Radiology)4;
Jennifer LS Khoo, FRCR, FHKAM (Radiology)4;
KW Tang, FRCR, FHKAM (Radiology)1
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Department of Radiology, Kwong Wah Hospital, Yaumatei, Hong Kong
3 Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Tuen Mun, Hong Kong
4 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
# Currently at Department of Radiology, Princess Margaret Hospital,
Laichikok, Hong Kong
Corresponding author: Dr Elaine WS Fok (elainefokws@gmail.com)
Abstract
Objective: To identify specific angiographic factors
associated with haemorrhagic presentation of brain
arteriovenous malformation in Chinese paediatric
patients.
Design: Retrospective cross-sectional observational
study.
Setting: Four locoregional tertiary neurosurgical
centres in Hong Kong: Queen Elizabeth Hospital,
Tuen Mun Hospital, Kwong Wah Hospital, and
Pamela Youde Nethersole Eastern Hospital.
Patients: Patients aged 18 years or younger who
underwent pretreatment digital subtraction
angiography for brain arteriovenous malformation
between 1 January 2005 and 31 July 2013 were
included. Patients were divided into haemorrhagic
and non-haemorrhagic groups based on the initial
presentation. Pretreatment digital subtraction
angiographies were independently reviewed by two
experienced neuroradiologists.
Main outcome measures: The following
parameters were evaluated for their association
with haemorrhagic presentation by univariate and
multivariate analyses: nidus location, nidus size,
nidus morphology (diffuse or compact); origin and
number of arterial feeders; venous drainage; number
of draining veins; presence of aneurysms, venous
varices, and venous stenosis.
Results: A total of 67 children and adolescents
(28 male, 39 female) with a mean age of 12 years
were included. Of them, 52 (78%) presented with
haemorrhage. Arteriovenous malformation size
(P=0.004) and morphology (P=0.05) were found
to be associated with haemorrhagic presentation
by univariate analysis. Small arteriovenous
malformation nidus size and diffuse nidal
morphology were identified as independent risk
factors for haemorrhage by multivariate analysis.
Conclusion: Smaller arteriovenous malformation
size and diffuse nidal morphology are angiographic
factors independently associated with haemorrhagic
presentation. Bleeding risk is important in
determining the therapeutic approach (aggressive
vs conservative) and timeframe, particularly in
paediatric patients.
New knowledge added by this
study
- Studies on paediatric arteriovenous malformation (AVM) are scarce and mostly based in Caucasian populations. This multicentre study involving Chinese paediatric patients found that small AVM nidus size and diffuse nidal morphology are independent risk factors for haemorrhage.
- These two angiographic features associated with haemorrhagic presentation can help local clinicians to assess bleeding risk and determine the therapeutic approach (aggressive vs conservative) and treatment timeframe in paediatric patients with cerebral AVM.
Introduction
Brain arteriovenous malformation (AVM) is a
vascular abnormality that consists of multiple
fistulous connections between arteries and veins
without a normal intervening capillary bed. It is
believed to be congenital in nature, and commonly
presents in early adulthood.1 The usual clinical
presentations of brain AVM include haemorrhage,
seizures, headache, and progressive neurological
deficit. About 52% to 77% of patients with AVM have
initial haemorrhagic presentation,2 3 4 which is also
associated with poorer prognosis. Various studies
evaluating the history of AVM record an annual
haemorrhage rate of about 2% to 4%.1 5
Computed tomography (CT) is the initial
screening tool for identifying haemorrhage and
demonstrating the location of the AVM. Subsequent
angiographic evaluation is required for virtually
all patients with suspected AVM, with digital
subtraction angiography (DSA) being accepted as the
gold standard for characterisation and grading. The
information obtained from the angiogram is crucial
in treatment decision-making and prognostication.
Brain AVM is an important cause of
haemorrhagic stroke in children.6 7 8 Studies in
adults have identified radiological features that are
associated with haemorrhagic presentation and
future haemorrhage.9 10 11 Similar studies on AVM in children are, however, scarce and mostly based
on studies from Europe and North America.12 13
Whether those angiographic features that predict
haemorrhage in Caucasian children with AVM
similarly predict haemorrhage in Chinese children
with AVM is unknown.
The objective of this multicentre study was to
determine specific angiographic factors associated
with haemorrhagic presentation in brain AVM in the
Hong Kong Chinese paediatric population, with a
view to assisting clinical decision-making regarding
the optimal timing and type of treatment.
Methods
This was a multicentre retrospective cross-sectional
observational study. We included patients aged
18 years or younger (at time of diagnosis) who
underwent pretreatment cerebral DSA for a principal
diagnosis of brain AVM from 1 January 2005 to 31
July 2013.
Patients were recruited from four locoregional
tertiary neurosurgical centres in Hong Kong: Queen Elizabeth
Hospital, Tuen Mun Hospital, Kwong Wah Hospital,
and Pamela Youde Nethersole Eastern Hospital.
These are the major acute hospitals belonging to the
catchment areas of Kowloon Central, New Territories
West, Kowloon West, and Hong Kong East clusters,
respectively, according to the geographical cluster
designation by the Hospital Authority. These
clusters serve approximately 4 million Hong Kong
inhabitants. Consecutive patients were retrieved
from the Clinical Data Analysis and Reporting
System by entering the targeted date range (01
January 2005 to 31 July 2013, inclusive) and the
following search parameters: age range (0-18 years);
International Classification of Diseases, 9th Revision,
diagnostic code (747.81, AVM); and procedure code
(88.41, arteriography of cerebral arteries). Exclusion
criteria included a lack of accessible pretreatment
DSA, other angiographic diagnoses (eg spinal AVM,
vein of Galen aneurysmal malformation, dural
arteriovenous fistulae), and non-Chinese ethnicity
based on data extracted from the electronic Patient
Record (ePR) and radiology reports. Approval was
obtained from the institutional ethics committee
and patient consent was waived for this retrospective
study.
Basic demographic factors, including age
at presentation and clinical symptoms, were
obtained from the ePR. Patients were divided into
a haemorrhagic group (those presenting with
intracranial haemorrhage) and a non-haemorrhagic
group based on the CT of the brain at presentation.
Pretreatment DSAs were independently reviewed by
two experienced interventional neuroradiologists
(with 7 years and 15 years of experience) who were
blinded to the clinical presentation and provided
with the same demographic data. Each brain AVM
was evaluated for the following parameters: nidus
location (deep: thalamus, basal ganglia, corpus
callosum, or brain stem vs hemispheric: cerebral or
cerebellar lobes), nidus size (small <3 cm vs medium
3-6 cm vs large >6 cm), nidus morphology (compact:
little or no intervening brain within the nidus vs
diffuse: presence of significant intervening brain
within the nidus) [Figs 1 and 2], origin of arterial
feeders (cortical vs deep), number of arterial feeders
(single vs multiple), presence of either flow-related
or intranidal aneurysms (yes vs no), venous drainage
destination (superficial vs deep), number of draining
veins (single vs multiple), presence of venous varices
(yes vs no), and presence of venous stenosis (yes vs
no). Any discrepancy in reviews between the two
neuroradiologists was resolved by mutual consensus.
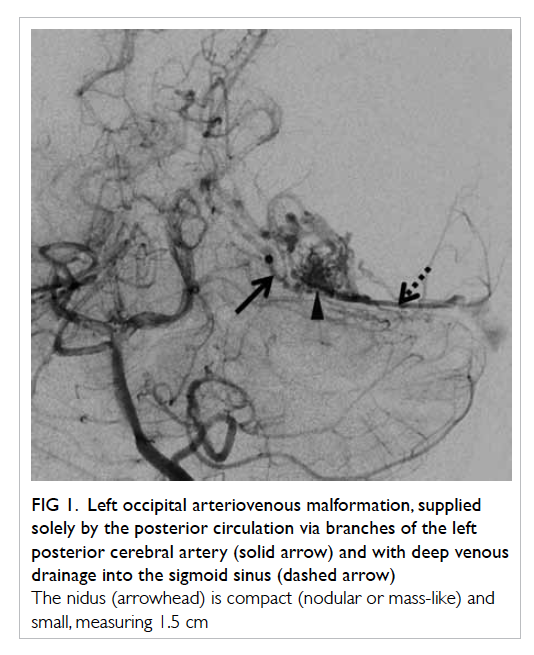
Figure 1. Left occipital arteriovenous malformation, supplied solely by the posterior circulation via branches of the left posterior cerebral artery (solid arrow) and with deep venous drainage into the sigmoid sinus (dashed arrow)
The nidus (arrowhead) is compact (nodular or mass-like) and small, measuring 1.5 cm
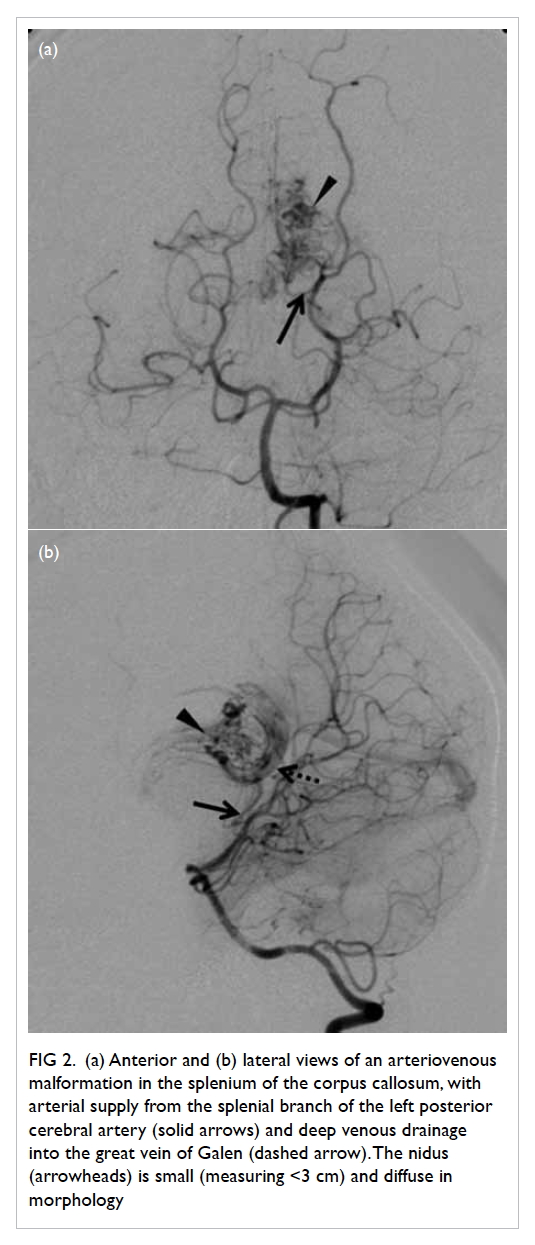
Figure 2. (a) Anterior and (b) lateral views of an arteriovenous malformation in the splenium of the corpus callosum, with arterial supply from the splenial branch of the left posterior cerebral artery (solid arrows) and deep venous drainage into the great vein of Galen (dashed arrow). The nidus (arrowheads) is small (measuring <3 cm) and diffuse in morphology
Association between the angiographic features
and haemorrhage was analysed using Chi squared
test and Fisher’s exact test for categorical variables, and
Student’s t test for numerical variables in univariate
analysis. Logistic regression (with “enter” strategy)
was carried out for covariates with a P value of <0.15.
All statistical calculations were performed using the
Statistical Package for the Social Sciences (Windows
version 16.0; SPSS Inc, Chicago [IL], US).
Results
The sample included 67 children and adolescents
who were eligible for inclusion, of which 28 (42%)
were boys and 39 (58%) were girls. Among the
patients, 52 (78%) were in the haemorrhagic group
and 15 (22%) were in the non-haemorrhagic group.
The mean age at presentation was 12 years (range, 2-18
years). No significant differences in age (P=0.15)
or sex (P=0.88) were demonstrated between the
haemorrhagic and non-haemorrhagic groups. Of
the 67 patients, one in the haemorrhagic group
was known to have idiopathic thrombocytopenic
purpura. The remaining 66 patients had no known
medical condition predisposing to intracranial
haemorrhage.
Of the 67 children, 25 (37%) presented with
headache, 12 (18%) with hemiplegia, 11 (16%) with
convulsion, seven (10%) with collapse, three (4%) with loss
of consciousness, one (1%) with cerebellar signs, and
eight (12%) had other features, including confusion,
decreased responsiveness, numbness, and restricted
ocular motion. There were more asymptomatic
patients in the non-haemorrhagic group (Fig 3). Three patients, all of whom were in the non-haemorrhagic
group, were diagnosed incidentally
with AVM during examination for precocious
puberty, scalp haemangioma, and suspected
neurofibromatosis type 1.
The frequency of haemorrhage of the 67
patients as a function of angioarchitectural features
is shown in Table 1.
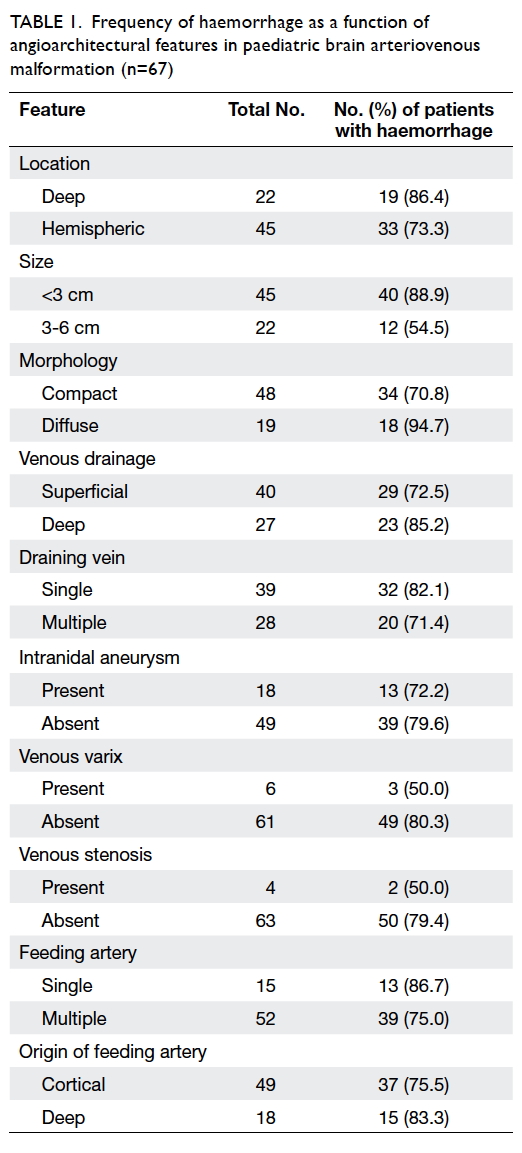
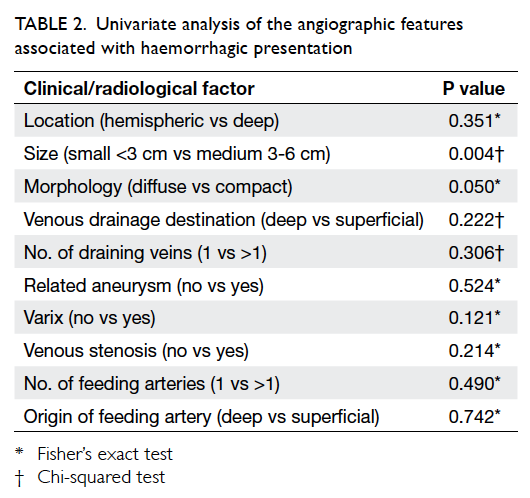
Table 1. Frequency of haemorrhage as a function of angioarchitectural features in paediatric brain arteriovenous malformation (n=67)
After univariate analysis, AVM size
(P=0.004) and morphology (P=0.05)
were the two factors found to be significantly
associated with haemorrhagic presentation (Table 2). After multivariate analysis, small AVM size and diffuse nidal morphology were identified as
independent risk factors for haemorrhage (Table 3).
The odds of haemorrhagic presentation in patients
with small AVM was about 9 times that of patients
with medium-size AVM, whereas the odds for
haemorrhagic presentation in patients with diffuse
nidal morphology was approximately 12 times that of
patients with compact AVM morphology. Factors
found not to be statistically significantly associated
with haemorrhagic presentation included location,
origin of arterial feeders, number of arterial feeders,
presence of related aneurysms, venous drainage,
number of draining veins, presence of venous
varices, and presence of venous stenosis.
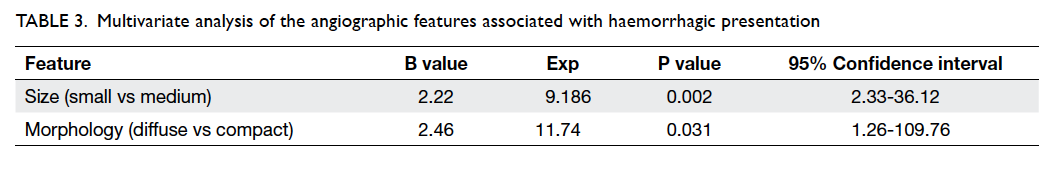
Table 3. Multivariate analysis of the angiographic features associated with haemorrhagic presentation
Discussion
To date, DSA remains the gold standard for evaluating
brain AVM owing to its superior temporal and spatial
resolution, with the ability to provide dynamic
information and allow accurate identification
of supplying arteries and draining veins. Generally
CT and magnetic resonance angiography studies do
not provide important dynamic information on the
arterial supply and venous drainage.
Current treatment approaches for brain
AVM include microsurgical resection, stereotactic
radiosurgery, embolisation, a combination of these
methods, and watchful waiting. As each patient with
AVM is different, there is no universal algorithm or
protocol to be followed. The management of brain
AVM is highly individualised, requiring careful
consideration of multiple factors, including lesion-related
factors (eg size, location, and configuration) which can be obtained from cerebral angiogram;
patient-related factors (eg life expectancy, general
health, and lifestyle); and treatment-related risks.
Children with AVM pose a particular problem in
that they have a high cumulative bleeding risk due to
their young age at presentation, and any insult to the
developing brain (either spontaneous haemorrhage
or treatment-related morbidity) may have lifelong
and profound sequelae.14
To the best of our knowledge, our study is
the first to evaluate the risk factors for brain AVM
haemorrhage in Chinese paediatric patients. Ethnic
differences exist in the incidence and haemorrhagic
risk of AVM, and data from western populations
are not routinely generalisable to the Chinese
population. A cohort study of 1028 adult patients
with AVM in the US has established a
role of ethnic differences in brain AVM, with excess
incidence in Asians, blacks, and Hispanics compared
with Caucasians.15 The analysis reveals a statistically
significant increased risk for subsequent AVM
haemorrhage among Hispanics compared with
Caucasians, and an insignificant trend for blacks and
Asians.
Studies in adults have identified specific
angiographic features of AVM that are associated
with haemorrhagic presentation and future bleeding,
including small size (<3 cm), deep location, deep
venous drainage, single draining vein, intranidal
aneurysms, and associated venous ectasia or
stenosis.5 14 16 17
Our study identified small AVM size (<3 cm)
to be an independent risk factor for haemorrhage.
Small AVM size has been identified as a risk factor
for haemorrhage in multiple adult studies,5 18 19 20
which is also demonstrated in a western
paediatric population.12 Although the underlying
pathophysiological mechanism is uncertain, some
authors have postulated a relationship between AVM
size and feeding artery pressures.8 12 Spetzler et al17
found a higher rate of haemorrhagic presentation
among smaller AVMs and noted that smaller AVMs
were associated with higher feeding artery pressures
at the time of surgical management as well as larger
haematoma sizes.
Diffuse AVM nidal morphology was identified
as another independent risk factor for haemorrhage
in our study. Although a similar relationship between
morphology and haemorrhage was not demonstrated
in a Caucasian paediatric population,12 diffuse AVM
nidal morphology has been demonstrated in adults
as a risk factor for haemorrhage.21 The underlying
pathophysiological mechanism is uncertain. More
information is needed to determine whether diffuse
morphology is associated with haemodynamic
aberrations such as increased pressure in the feeding
artery or draining vein to account for the observed
increased risk of haemorrhage.
Our study has several limitations. First,
owing to the retrospective nature of this study,
AVM patients with poorer clinical presentation
who are unfit for DSA were not included. Second,
although this is a multicentre study, the sample size
was relatively small owing to the small number of
paediatric patients undergoing DSA for AVM. Our
study has also underestimated the haemorrhagic
proportion of the study population, thus any
potential associations between other angiographic
features with haemorrhagic presentation that are
more subtle to detect would remain undetected.
Third, variations exist in the quality and amount
of available angiographic images, as well as in the
level of experience of the angiographers among the
various centres; these may affect the radiological
interpretation. Presence of intracranial haemorrhage
can be inferred from the pretreatment DSA due to
presence of blood vessel displacement, which is an
inherent limitation of this study. Fourth, as presence
of haemorrhage may render an originally compact
nidus into a diffuse morphology, this is a limiting
factor in determining the association between
diffuse morphology and haemorrhagic presentation.
Fifth, we were unable to control for the timing of
DSA following the onset of presentation owing to the
retrospective nature of this study. Lastly, the extent
to which certain angiographic risk factors existent
at the time of haemorrhagic presentation can be
extrapolated as predictors of future haemorrhage in
AVM is controversial. In other words, factors present
at the time of presentation are not necessarily
accurate predictors of future risk. For instance,
several adult-based studies have identified a higher
incidence of haemorrhagic presentation in small
AVMs, but failed to find an association between
AVM size and future haemorrhage.22 23
Unlike in adults, large-scale prospective
studies aiming to study the natural course of
paediatric AVMs are unlikely to take place owing to
the relatively strong argument against conservative
treatment, according to the prevailing view that
ruptured paediatric AVMs should be treated
aggressively owing to the significant risk of recurrent
haemorrhage and subsequent morbidity and
mortality.12 24 The recent controversial ARUBA (A Randomised trial of Unruptured Brain Arteriovenous
malformations) in adults has demonstrated that
medical management alone is superior in patients
with unruptured AVMs,25 but there is insufficient
scientific evidence to justify extrapolation of these
results to a paediatric population. Moreover, while
it has been shown that paediatric AVMs with
haemorrhagic presentation do not necessarily have
a higher risk of future haemorrhage nor a higher
annualised bleeding risk than adults,26 their greater
cumulative risk given their longer remaining life
expectancy may be an argument for more aggressive
treatment of paediatric AVMs. Choice of treatment
for a small, unruptured paediatric AVM is therefore
complex and should involve thorough consideration
of other angioarchitectural factors on a case-by-case
basis.
Despite these limitations, our study provides
useful initial insights to the angiographic features
associated with haemorrhagic presentation of
AVMs in Chinese paediatric patients from multiple
locoregional neurosurgical centres. These features
may assist in stratifying risk of haemorrhage and
assign priority for intervention, although data from
future larger-scale studies may be needed before such
features can be robustly applied as haemorrhagic
risk predictors in Chinese children with AVM.
References
1. Ondra SL, Troupp H, George ED, Schwab K. The natural
history of symptomatic arteriovenous malformations of
the brain: a 24-year follow-up assessment. J Neurosurg
1990;73:387-91. Crossref
2. Choi JH, Mohr JP. Brain arteriovenous malformations in
adults. Lancet Neurol 2005;4:299-308. Crossref
3. Freidlander RM. Clinical practice. Arteriovenous
malformations of the brain. N Engl J Med 2007;356:2704-12. Crossref
4. Hofmeister C, Stapf C, Hartmann A, et al. Demographic,
morphological, and clinical characteristics of 1289
patients with brain arteriovenous malformation. Stroke
2000;31:1307-10. Crossref
5. Graf CJ, Perret GE, Torner JC. Bleeding from cerebral
arteriovenous malformations as part of their clinical
history. J Neurosurg 1983;58:331-7. Crossref
6. Jordan LC, Hillis AE. Hemorrhagic stroke in children.
Pediatr Neurol 2007;36:73-80. Crossref
7. Meyer-Heim AD, Boltshauser E. Spontaneous intracranial
haemorrhage in children: aetiology, presentation and
outcome. Brain Dev 2003;25:416-21. Crossref
8. Giroud M, Lemesle M, Madinier G, Manceau E, Osseby
GV, Dumas R. Stroke in children under 16 years of age.
Clinical and etiological difference with adults. Acta Neurol
Scand 1997;96:401-6. Crossref
9. Stefani MA, Porter PJ, terBrugge KG, Montanera W,
Willinsky RA, Wallace MC. Angioarchitectural factors
present in brain arteriovenous malformations associated
with hemorrhagic presentation. Stroke 2002;33:920-4. Crossref
10. Turjman F, Massoud TF, Viñuela F, Sayre JW, Guglielmi G,
Duckwiler G. Correlation of the angioarchitectural features
of cerebral arteriovenous malformations with clinical
presentation of hemorrhage. Neurosurgery 1995;37:856-60; discussion 860-2. Crossref
11. Nataf F, Meder JF, Roux FX, et al. Angioarchitecture
associated with haemorrhage in cerebral arteriovenous
malformations: a prognostic statistical model.
Neuroradiology 1997;39:52-8. Crossref
12. Ellis MJ, Armstrong D, Vachhrajani S, et al.
Angioarchitectural features associated with hemorrhagic
presentation in pediatric cerebral arteriovenous
malformations. J Neurointerv Surg 2013;5:191-5. Crossref
13. Di Rocco C, Tamburrini G, Rollo M. Cerebral arteriovenous
malformations in children. Acta Neurochir (Wien)
2000;142:145-56; discussion 156-8. Crossref
14. Fleetwood IG, Steinberg GK. Arteriovenous malformations.
Lancet 2002;359:863-73. Crossref
15. Kim H, Stephen S, McChulloch, et al. Racial/ethnic
differences in longitudinal risk of intracranial hemorrhage
in brain arteriovenous malformation patients. Stroke
2007;38:2430-7. Crossref
16. Farhat HI. Cerebral arteriovenous malformations. Dis Mon
2011;57:625-37. Crossref
17. Spetzler RF, Hargraves RW, McCormick PW, Zabramski
JM, Flom RA, Zimmerman RS. Relationship of perfusion
pressure and size to risk of hemorrhage from arteriovenous
malformations. J Neurosurg 1992;76:918-23. Crossref
18. Crawford PM, West CR, Chadwick DW, Shaw MD.
Arteriovenous malformations of the brain: natural history
in unoperated patients. J Neurol Neurosurg Psychiatry
1986;49:1-10. Crossref
19. Guidetti B, Delitala A. Intracranial arteriovenous
malformations. Conservative and surgical treatment. J
Neurosurg 1980;53:149-52. Crossref
20. Itoyama Y, Uemura S, Ushio Y, et al. Natural course of
unoperated intracranial arteriovenous malformations:
study of 50 cases. J Neurosurg 1989;71:805-9. Crossref
21. Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ,
Kondziolka D. Factors that predict the bleeding risk of
cerebral arteriovenous malformations. Stroke 1996;27:1-6. Crossref
22. da Costa L, Wallace MC, Ter Brugge KG, O’Kelly C,
Willinsky RA, Tymianski M. The natural history and
predictive features of hemorrhage from brain arteriovenous
malformations. Stroke 2009;40:100-5. Crossref
23. Stapf C, Mast H, Sciacca RR, et al. Predictors of
hemorrhage in patients with untreated brain arteriovenous
malformation. Neurology 2006;66:1350-5. Crossref
24. Blauwblomme T, Bourgeois M, Meyer P, et al. Long-term
outcome of 106 consecutive pediatric ruptured brain
arteriovenous malformations after combined treatment.
Stroke 2014;45:1664-71. Crossref
25. Mohr JP, Parides MK, Stapf C, et al. Medical management
with or without interventional therapy for unruptured brain
arteriovenous malformations (ARUBA): a multicentre,
non-blinded, randomised trial. Lancet 2014;383:614-21. Crossref
26. Fullerton HJ, Achrol AS, Johnston SC, et al. Long-term
hemorrhage risk in children versus adults with brain
arteriovenous malformations. Stroke 2005;36:2099-104. Crossref