Hong Kong Med J 2015 Oct;21(5):389–93 | Epub 31 Jul 2015
DOI: 10.12809/hkmj144481
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Paracetamol overdose in Hong Kong: is the 150-treatment line good enough to cover patients
with paracetamol-induced liver injury?
Simon TB Chan, MB, BS1;
CK Chan, Dip Clin Tox, FHKAM (Emergency Medicine)2;
ML Tse, FHKCEM, FHKAM (Emergency Medicine)2
1 Department of Accident and Emergency, United Christian Hospital, Kwun Tong, Hong Kong
2 Hong Kong Poison Information Centre, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr Simon TB Chan (ctb021@ha.org.hk)
Abstract
Objectives: To evaluate the failure rate of the
150-treatment line for paracetamol overdose in Hong
Kong, and the impact if the treatment threshold was
lowered.
Design: Case series.
Setting: Public hospitals, Hong Kong.
Patients: All patients with acute paracetamol
overdose reported to the Hong Kong Poison
Information Centre from 1 January 2011 to 31
December 2013 were studied and analysed for
the timed serum paracetamol concentration and
their relationship to different treatment lines. Presence
of significant liver injury following paracetamol
overdose was documented. The potential financial
burden of different treatment lines implemented
locally was estimated.
Results: Of 893 patients, 187 (20.9%)
had serum paracetamol concentration above
the 150-treatment line, 112 (12.5%) had serum
paracetamol concentration between the 100- and 150-treatment lines, and 594 (66.5%) had
serum paracetamol level below the 100-treatment
line. Of the 25 (2.8%) patients who developed
significant liver injury, two were between the 100- and 150-treatment lines, and the other two were
below the 100-treatment line. The failure rate of
the 150-treatment line was 0.45%. Lowering the
treatment threshold to the 100-treatment line might
lower the failure rate of the treatment nomogram
to 0.22% but approximately 37 more patients per
year would need to be treated. It would incur an
additional annual cost of HK$189 131 (US$24 248), and an
additional 1.83 anaphylactoid reactions per year. The
number needed-to-treat to potentially reduce one
significant liver injury is 112.
Conclusions: Lowering the treatment threshold of
paracetamol overdose may reduce the treatment-line failure rate. Nonetheless such a decision
must be balanced against the excess in treatment
complications and health care resources.
New knowledge added by this study
- For paracetamol overdose in Hong Kong, the failure rate of the 150-treatment line is 0.45%. Lowering the treatment threshold to 100-treatment line may lower the failure rate to 0.22%.
- Implementing the 100-treatment line in Hong Kong would incur an annual cost of HK$189 131 (US$24 248), 37 more patients per year needing treatment, and an additional 1.83 anaphylactoid reactions per year. The number needed-to-treat to potentially reduce one significant liver injury is 112.
- Clinicians should be aware of the chance of treatment-line failure in patients with acute paracetamol overdose.
- Recommendations for the treatment threshold for acute paracetamol overdose may be evaluated together with the clinical and financial impacts described in this study.
Introduction
Paracetamol is a common analgesic and antipyretic,
and is currently the most commonly overdosed
therapeutic agent in Hong Kong, accounting for 8.4%
of all poisoning cases.1 After the first human case
report of paracetamol-induced liver injury in 1966,2
paracetamol overdose remains an important cause
of acute liver failure with mortalities worldwide. The
efficacy of N-acetylcysteine (NAC) in the treatment
and prevention of paracetamol-induced liver injury
has been established over the last decades.3 4 In acute paracetamol overdose, serum paracetamol
concentration measured at 4 to 24 hours post-ingestion
(timed serum paracetamol concentration)
is plotted on the Rumack-Matthew nomogram.
Treatment with NAC should be initiated if the
serum paracetamol concentration is plotted on or
above the treatment line.5 Different treatment lines
exist, however, and there is no worldwide consensus
on the safest serum paracetamol concentration at
which to initiate NAC treatment.
In the United States, the timed serum
paracetamol concentration is plotted against a
single treatment line starting at 150 mg/L at 4 hours
post-ingestion (150-treatment line).6 Patients with
serum paracetamol concentration above this line
are treated with NAC. This line was imposed by the
United States Food and Drug Administration with a
25% safety margin based on the work of Rumack in
1975,5 and was also adopted in Australia and New
Zealand.7
Previously in the United Kingdom, two
different treatment lines were used. Patients were
categorised into ‘normal risk’ or ‘high risk’ according
to their medical and behavioural background.
Those patients with possible glutathione depletion,
for example with malnutrition or chronic heavy
alcoholism, were considered ‘high risk’ while the
remaining patients were considered ‘normal risk’.
Treatment lines starting at 200 mg/L (200-treatment
line) and 100 mg/L (100-treatment line) at 4 hours
post-ingestion were used in patients with ‘normal
risk’ and ‘high risk’, respectively.
The Medicines and Healthcare products
Regulatory Agency of the United Kingdom lowered
the threshold of NAC treatment in 2012,8 following a
series of patients with severe liver injury and several
mortalities after paracetamol overdose were reported
to have a serum paracetamol concentration below the
level dictated by the previous treatment protocol.9
All patients in the United Kingdom with serum
paracetamol concentration over the 100-treatment
line are now prescribed NAC treatment.
The Hong Kong Poison Information Centre
(HKPIC) currently recommends intravenous NAC
treatment in patients with serum paracetamol
concentration above the 150-treatment line. One
course of NAC treatment typically takes 21 hours
of intravenous infusion in hospital under close
observation. In this study, we examined a series
of non-staggered acute paracetamol overdose
cases in Hong Kong to determine how well the
current 150-treatment line can cover patients with
paracetamol-induced liver injury and the impact
on the local health care system if the treatment
threshold is changed.
Methods
We performed a retrospective observational study by
reviewing patients with acute paracetamol overdose
who presented to 16 emergency departments (EDs)
in public hospitals in Hong Kong between 1 January
2011 and 31 December 2013. Data were retrieved
from the electronic database of the HKPIC and
electronic Patient Record of the Hospital Authority.
All patients aged 12 years or above with acute
paracetamol overdose were included.
In this study, acute paracetamol overdose was
defined as ingestion of paracetamol or paracetamol-containing
medications in a single attempt. As some
patients intentionally took a large number of tablets,
we allowed a maximum duration of ingestion
process for up to 1 hour. When the ingestion process
occurred over 1 hour, the overdose was
considered to be staggered and such patients were
excluded from the study. Patients were also excluded
if time of ingestion was undetermined, no serum
paracetamol concentration was available within 4
to 24 hours post-ingestion, or patients presented to
EDs more than 24 hours post-ingestion.
The following data were collected: clinical
profile including age, sex, first serum paracetamol
concentration between 4 and 24 hours post-ingestion,
treatment given, and the presence of significant liver
injury during the episode. Significant liver injury was
defined as serum alanine aminotransferase (ALT)
level of ≥1000 IU/L in the absence of a known history
of deranged liver function.
Results
There were a total of 1243 patients with acute
paracetamol overdose within the study period.
Exclusions included 73 patients with staggered
overdose, 137 patients with undetermined time of
paracetamol ingestion, 100 patients with no serum
paracetamol concentration available within 4 to 24
hours of ingestion, and 40 patients who presented
to the EDs of >24 hours post-ingestion. Data on 893
patients who fulfilled the inclusion criteria were
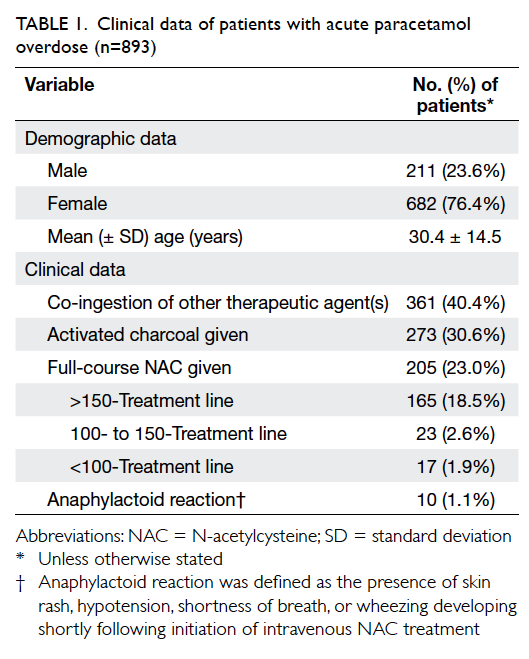
analysed. The clinical data of the studied patients are
presented in Table 1.
No deaths occurred in the study population
and no patient required liver transplantation.
There were 187 (20.9%) patients with a serum
paracetamol concentration above the 150-treatment
line, 112 (12.5%) patients had a serum paracetamol
concentration between the 100- and 150-treatment
lines, and the remaining 594 (66.5%) patients
had serum paracetamol concentration below the
100-treatment line.
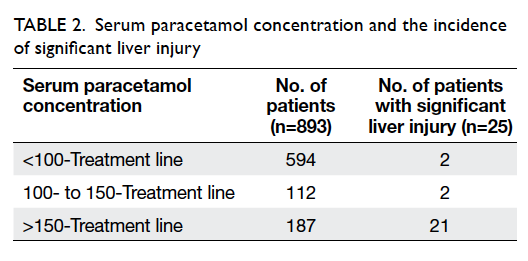
Significant liver injury occurred in 25 patients
within the study period, giving an overall incidence of 2.8% following acute paracetamol
overdose (Table 2). Four patients with
serum paracetamol concentration below the
150-treatment line developed significant liver injury.
The failure rate of the 150-treatment line was 0.45%
(4/893). If 100-treatment line is applied instead
of 150-treatment line, two patients with serum
paracetamol concentration below the 100-treatment
line developed significant liver injury. The failure
rate of the 100-treatment line would thus be 0.22%
(2/893).
Discussion
Paracetamol overdose is a commonly encountered
problem in Hong Kong, and is the single most
common cause of poisoning. According to the
Rumack-Matthew nomogram, a serum paracetamol
concentration starting from 200 mg/L at 4 hours
is associated with an increased risk of liver injury
and death.10 The clinical decision to commence
treatment with NAC is dictated by the timed serum
paracetamol concentration plotted against the
nomogram with a treatment line. If the timed serum
paracetamol concentration is above the treatment
line, NAC treatment is indicated. Based on different
considerations, for example the accuracy of clinical
history and individual susceptibility, different
treatment lines are applied by different countries or
centres to guide initiation of NAC treatment.
Since the United Kingdom lowered the NAC
treatment threshold to the 100-treatment line,8 there
has been discussion in Hong Kong about the benefit
of changing local recommendations. Nonetheless,
it is unknown whether the reasons for changing
the recommendation in the United Kingdom apply
to the Hong Kong population. This study serves to
provide more information for further discussion and
consideration.
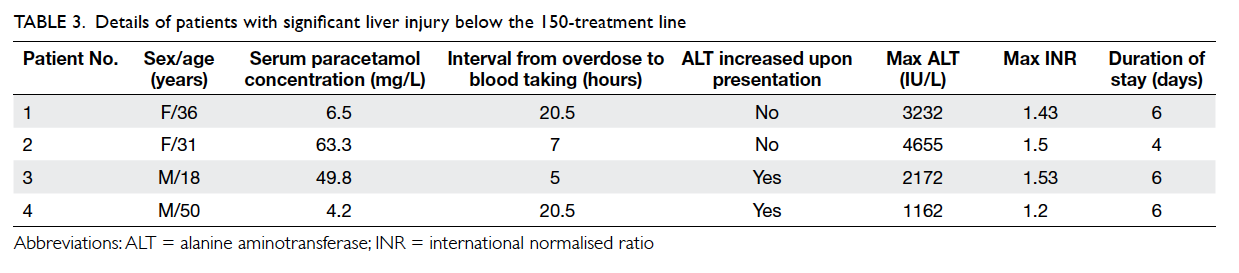
We were most interested to identify patients
who developed significant liver injury following
paracetamol overdose in which the serum
paracetamol concentration was lower than the
treatment line. In the 3-year study period, four
patients with a paracetamol concentration below
the 150-treatment line developed significant liver
injury. These cases are discussed in detail below and
summarised in Table 3.
Case 1
A 36-year-old woman with known depressive
disorder attended the ED 20.5 hours following
ingestion of 100 tablets of paracetamol. She had
abdominal pain afterwards. On presentation,
the paracetamol concentration was 43 µmol/L
(6.5 mg/L). This level falls between the 100- and
150-treatment lines. The level of ALT was 53 IU/L
and international normalised ratio (INR) was 1.03.
She was admitted to the medical ward. Although
her serum paracetamol concentration plotted below
the 150-treatment line, NAC treatment was given
soon after admission based on the clinical features
of hepatitis. Her liver enzyme level started to elevate
the next day with clinical jaundice, and on day 2 of
admission her ALT level peaked at 3232 IU/L, and
INR at 1.43 with bilirubin level of 45 µmol/L. She was
treated conservatively and liver function gradually
improved. She was discharged on day 6 of admission.
Case 2
A 31-year-old woman attempted suicide by ingesting
around 50 tablets of over-the-counter drugs. She was
brought to the ED 3 hours afterwards and complained
of mild dizziness and nausea. Activated charcoal
50 g was given and she was admitted to the Intensive
Care Unit (ICU). Serum paracetamol concentration
taken at 7 hours post-ingestion was 418 µmol/L
(63.3 mg/L), between the 100- and 150-treatment
lines. The patient was treated supportively in the
ICU for the first day. At 24-hour post-ingestion her
ALT level was 48 IU/L, INR was 1.3, and at 29 hours
post-ingestion the ALT level elevated to 73 IU/L,
with INR of 1.4. Intravenous NAC treatment was
commenced 30 hours post-ingestion. Liver function
continued to deteriorate: ALT level peaked at 4655 IU/L
and INR peaked at 1.5 on day 3 of admission. The
serum bilirubin level was 67 µmol/L. She had clinical
jaundice and vomiting. On day 4, NAC infusion was
stopped. She had full recovery of liver function at
1-month follow-up.
Case 3
An 18-year-old man ingested around 50 tablets of
paracetamol in a suicide attempt. He attended the ED
5 hours later. He was asymptomatic on presentation.
Serum paracetamol concentration at 5 hours post-ingestion
was 330 µmol/L (49.8 mg/L), below the
100-treatment line. At presentation his ALT level was 64 IU/L
and INR was 1.06. Liver function tests repeated
at 7 hours post-ingestion showed ALT level was
increased to 98 IU/L and INR was 1.3. Intravenous
NAC was started. The liver function deterioration
peaked at day 3 of admission with ALT level of
2172 IU/L and INR of 1.53 and the patient complained
of abdominal pain; NAC infusion was continued
until day 5 of admission. His liver function improved
and he was transferred to the psychiatric ward for
management of depression on day 6. He had full
recovery of his liver function.
Case 4
A 50-year-old man had flu-like symptoms and fever
for 5 days. He ingested 60 tablets of paracetamol in a
suicide attempt related to financial stress and physical
discomfort. At 20 hours after ingestion, he attended
the ED for recurring fever. On presentation his body
temperature was 40.2°C. Blood tests revealed ALT
level of 511 IU/L, INR of 1.1, and serum paracetamol
concentration of 28 µmol/L (4.2 mg/L), below the
100-treatment line at 20.5 hours; NAC treatment
was given based on the clinical features of chemical
hepatitis. His liver markers peaked the next day
with ALT level of 1162 IU/L, INR of 1.2, and with
abdominal pain. His fever and respiratory symptoms
subsided with a course of intravenous antibiotics.
His liver function gradually improved and he was
transferred to the psychiatric ward on day 6 after
admission.
All four patients were considered ‘normal risk’
according to the old United Kingdom classification
of treatment line options.
Analysis of these four cases revealed that if
the NAC treatment threshold had been lowered
from the 150-treatment line to 100-treatment line,
the treatment line would have covered the first two
cases but not the last two. The treatment-line failure
rate would thus be reduced by half to 0.22%. A lower
failure rate of the treatment line implies that people
who will develop liver injury following paracetamol
overdose are more likely to receive early treatment
with NAC. Although not proven in this study, this
should prevent a small number of cases of significant
morbidity related to severe paracetamol poisoning.
Giving intravenous NAC is not without risk,
however. If the 100-treatment line had been applied
instead of the 150-treatment line, the number of
patients in this study in whom NAC treatment would
have been indicated would increase from 187 (20.9%)
to 299 (33.5%)—an additional 112 courses of NAC
over 3 years. In addition, since 4.9% of the patients
given NAC in this study developed an anaphylactoid
reaction, there would also have been an additional
5.49 anaphylactoid reactions (1.83 patients/year).
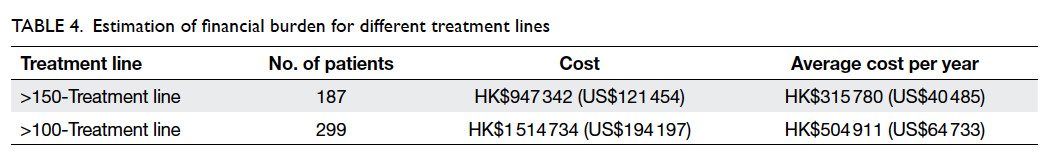
The financial burden of treating additional
patients with NAC courses can be estimated by
additional cost of hospital stay11 and drug cost
of NAC, approximately HK$5066 (US$650) per
standard 21-hour intravenous NAC administration.
This is similar to the estimated cost of a
standard NAC course in a United Kingdom study.12
An additional 112 courses of NAC would cost
HK$567 392 (US$72 743) within the study period of
3 years with an average of HK$189 131 (US$24 248)
per year. The calculations are shown in Table 4. On
rare occasions NAC treatment may be extended over
21 hours and result in a further increase in the actual
cost.
The liver injury in case 2 was judged to have
been preventable by timely NAC treatment in
nomogram perspective. Thus, we would need to
administer NAC to an additional 112 patients to
achieve potential benefit in one patient. The number
needed-to-treat of lowering 150-treatment line to
100-treatment line to potentially prevent one case of
paracetamol-induced liver injury is 112.
Despite these data, the clinical decision to
initiate NAC treatment may not depend solely
on the timed serum paracetamol concentration.
As illustrated in case 1, NAC treatments were
occasionally initiated based on the patient’s
presentation and doctor’s clinical judgement.
In our study, 23 of 112 patients with serum
paracetamol concentration plotted between 100- and 150-treatment lines were prescribed NAC for
similar reasons. Thus if the 100-treatment line is
used instead of the 150-treatment line, the actual
number of additional NAC treatment that would
have been needed is 89 (ie 112-23 cases).
Limitations
In the clinical management of paracetamol overdose
in Hong Kong, patients may be discharged if their
serum paracetamol level is below the treatment line
and there are no active clinical symptoms. Although
no patient in this study was readmitted for hepatitis,
there may have been others who developed chemical
hepatitis and who were not brought to our attention.
Thus the incidence of liver injury will have been
underestimated.
Conclusions
Neither the 150- nor 100-treatment line can fully
cover all patients who develop significant liver
injury following paracetamol overdose. The failure
rate of the treatment lines and potential financial
burden were studied. This serves as the basis for
future considerations of treatment in Hong Kong for
paracetamol overdose.
References
1. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information
Centre: Annual Report 2012. Hong Kong J Emerg Med
2013;20:371-81.
2. Davidson DG, Eastham WN. Acute liver necrosis following
overdose of paracetamol. Br Med J 1966;2:497-9. Crossref
3. Prescott LF, Illingworth RN, Critchley JA, Stewart MJ,
Adam RD, Proudfoot AT. Intravenous N-acetylcysteine:
the treatment of choice for paracetamol poisoning. BMJ
1979;2:1097-100. Crossref
4. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy
of oral N-acetylcysteine in the treatment of acetaminophen
overdose. Analysis of the national multicenter study (1976
to 1985). N Engl J Med 1988;319:1557-62. Crossref
5. Rumack BH. Acetaminophen hepatotoxicity: the first 35
years. J Toxicol Clin Toxicol 2002;40:3-20. Crossref
6. Rowden AK, Norvell J, Eldridge DL, Kirk MA. Updates on
acetaminophen toxicity. Med Clin North Am 2005;89:1145-59. Crossref
7. Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA;
Panel of Australian and New Zealand clinical toxicologists.
Guidelines for the management of paracetamol
poisoning in Australia and New Zealand—explanation
and elaboration. A consensus statement from clinical
toxicologists consulting to the Australasian poisons
information centres. Med J Aust 2008;188:296-301.
8. Paracetamol overdose: new guidance on use of intravenous
acetylcysteine. Commission on Human Medicines, United Kingdom. Available from: https://www.cas.dh.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=101488. Accessed 7 Jul 2015.
9. Beer C, Pakravan N, Hudson M, et al. Liver unit admission
following paracetamol overdose with concentrations below
current UK treatment thresholds. QJM 2007;100:93-6. Crossref
10. Rumack BH, Matthew H. Acetaminophen poisoning and
toxicity. Pediatrics 1975;55:871-6.
11. Head 140, Expenditure estimates, The 2014-15 Budget,
Hong Kong Special Administrative Region, People’s
Republic of China. Available from: http://www.budget.gov.hk/2014/eng/pdf/head140.pdf. Accessed 19 Nov 2014.
12. McQuade DJ, Dargan PI, Keep J, Wood DM. Paracetamol
toxicity: What would be the implications of a change in UK
treatment guidelines? Eur J Clin Pharmacol 2012;68:1541-7. Crossref