Hong Kong Med J 2015 Jun;21:224–31 | Epub 22 May 2015
DOI: 10.12809/hkmj144380
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Hospital Authority audit of the outcome of endoscopic resection of superficial upper
gastro-intestinal lesions in Hong Kong
Anthony YB Teoh, FRCSEd (Gen)1;
Philip WY Chiu, FRCSEd1;
SY Chan, FRCSEd2;
Frances KY Cheung, FRCSEd (Gen)3;
KM Chu, FRCSEd2;
SS Kao, FRCSEd4;
TW Lai, FRCSEd (Gen)5;
CW Lau, FRCSEd6;
Simon YK Law, FRCSEd2;
Canice TL Leung, FRCSEd (Gen)7;
WK Leung, FRCP8;
Daniel KH Tong, FRCSEd (Gen)2;
SH Tsang, FRACS9
1 Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
2 Department of Surgery, Queen Mary Hospital, The University of Hong
Kong, Hong Kong
3 Department of Surgery, Pamela Youde Nethersole Hospital, Hong Kong
4 Department of Surgery, Queen Elizabeth Hospital, Hong Kong
5 Department of Surgery, Prince Margaret Hospital, Hong Kong
6 Department of Surgery, Yan Chai Hospital, Hong Kong
7 Department of Surgery, North District Hospital, Hong Kong
8 Department of Medicine, Queen Marry Hospital, The University of Hong
Kong, Hong Kong
9 Department of Surgery, United Christian Hospital, Hong Kong
Corresponding author: Dr Anthony YB Teoh (anthonyteoh@surgery.cuhk.edu.hk)
Abstract
Objectives: To review the short-term outcome of endoscopic resection of superficial upper gastro-intestinal lesions in Hong Kong.
Design: Historical cohort study.
Setting: All Hospital Authority hospitals in Hong Kong.
Patients: This was a multicentre retrospective study
of all patients who underwent endoscopic resection
of superficial upper gastro-intestinal lesions between
January 2010 and June 2013 in all government-funded
hospitals in Hong Kong.
Main outcome measures: Indication of the
procedures, peri-procedural and procedural
parameters, oncological outcomes, morbidity, and
mortality.
Results: During the study period, 187 lesions in
168 patients were resected. Endoscopic mucosal
resection was performed in 34 (18.2%) lesions and
endoscopic submucosal dissection in 153 (81.8%)
lesions. The mean size of the lesions was 2.6
(standard deviation, 1.8) cm. The 30-day morbidity
rate was 14.4%, and perforations and severe
bleeding occurred in 4.3% and 3.2% of the patients,
respectively. Among patients who had dysplasia or
carcinoma, R0 resection was achieved in 78% and
the piecemeal resection rate was 11.8%. Lateral
margin involvement was 14% and vertical margin
involvement was 8%. Local recurrence occurred in
9% of patients and 15% had residual disease. The
2-year overall survival rate and disease-specific
survival rate was 90.6% and 100%, respectively.
Conclusion: Endoscopic mucosal resection and endoscopic submucosal dissection were introduced in low-to-moderate–volume hospitals with
acceptable morbidity rates. The short-term survival
was excellent. However, other oncological outcomes
were higher than those observed in high-volume
centres and more secondary procedures were
required.
New knowledge added by this
study
- Endoscopic mucosal resection and endoscopic submucosal dissection (ESD) were introduced in low-to-moderate–volume hospitals with acceptable morbidity rates and excellent short-term survival.
- Other oncological outcomes were higher than those observed in high-volume centres and more secondary procedures were required.
- Better education in recognition of early upper gastro-intestinal neoplasms and pre-ESD workup is required.
- Key personnel who perform ESD in individual hospitals should be identified for further advanced training.
- A minimal level of competence should be established before beginners perform the procedure independently.
Introduction
The use of endoscopic resection in the treatment
of superficial gastro-intestinal neoplasms is gaining
popularity worldwide.1 2 3 4 5 6 7 8 9 10 11 Endoscopic mucosal
resection (EMR) and endoscopic submucosal
dissection (ESD) are the key endoscopic methods
pioneered in Japan and Korea for resection of
these lesions.1 2 3 4 5 6 7 Early gastric or oesophageal
neoplasms are associated with a low rate of lymph
node metastasis and endoscopic resection of these
neoplasms has been shown to be associated with a
low rate of morbidity and high long-term survival.6 7 Nevertheless, the adoption of these techniques
outside high-volume countries has been slow, as
the recognition of early gastric and oesophageal
neoplasms is difficult and the procedures required
for endoscopic resection are technically demanding
and have a long learning curve.8 9 10 11 12 13 14
In Hong Kong, an increased awareness and
recognition of early gastro-intestinal neoplasms
have resulted in greater frequency of diagnosis of
these lesions. Hence advanced endoscopic resection
procedures such as EMR or ESD are also being
performed increasingly. However, the scope of these
services provided by government-funded hospitals
operated by the Hospital Authority of Hong Kong
has not been previously defined. In addition there
are limited data on the outcome of these advanced
endoscopic procedures outside high-volume centres
of Japan and Korea.
The aim of the current study was to perform
a region-wide audit of the short-term clinical
and oncological outcomes of EMR and ESD for
superficial upper gastro-intestinal neoplasms in all
government-funded hospitals in Hong Kong.
Methods
This was a Hong Kong–wide retrospective review
commissioned by the Hospital Authority of Hong
Kong of all patients who underwent EMR or ESD for
superficial upper gastro-intestinal lesions between
January 2010 and June 2013 in 12 government-funded
hospitals. Patients were identified using
the Clinical Data Analysis and Reporting System
(CDARS) based on their diagnostic and procedural
coding (9th edition of the International Classification
of Diseases). The CDARS is a computer-based
administration database that records all the
diagnostic and procedural coding of admitted
patients. Patient data were retrieved and reviewed
manually through the system. Data were recorded
for indication of the procedures, peri-procedural and
procedural parameters, oncological outcomes, and
morbidity and mortality. The study was performed
according to the Declaration of Helsinki.
Endoscopic mucosal resection or endoscopic submucosal dissection procedure
All patients who underwent EMR or ESD of the
oesophagus, stomach, and duodenum were included
in the study. To differentiate these patients from
those that received simple polypectomy, EMR was
defined as the act of performing mucosectomy
with prior injection of a cushioning fluid into the
submucosa to hasten removal of the lesions. It was
acknowledged that EMR could be performed by a
variety of methods but this review did not attempt
to sub-classify patients according to the different
techniques used.1 On the other hand, ESD was
defined as a more refined form of mucosectomy that
involved submucosal injection of a cushioning fluid,
circumferential incision of the mucosa, followed
by dissection of the submucosal tissue to free the
lesion away from surrounding tissue. The type of
endoscopic knives used for mucosal incision and
submucosal dissection were also recorded.
The outcomes of EMR and ESD were assessed clinically and oncologically.
Assessment of clinical outcomes
Compared with conventional polypectomy, ESD is a
technically demanding procedure that is associated
with an increased risk of complications. In particular,
the risk of perforation and bleeding are heightened
in inexperienced operators.13 14 In the current study, intra-procedural and post-procedural complications
were recorded and factors alluding to development
of these complications were also reviewed.
Assessment of oncological outcomes
To determine whether EMR or ESD can provide
adequate oncological clearance for neoplastic
lesions, assessment of long-term survival is essential.
However, since EMR or ESD procedures have been
performed in Hong Kong for only a short period of
time, such assessment was not possible. Thus, the
adequacy of oncological control of EMR or ESD was
based on completeness of resection as judged during
the procedure, presence of margin involvement
(lateral and deep), the need for piecemeal resection,
and recurrence rates. En-bloc resection was defined
as resection of the tumour in one piece. R0 resection
was defined as resection of the tumour with clear
lateral and vertical margins. R1 resection was defined
as microscopic involvement of the margins. R2
resection was defined as macroscopic involvement
of margins as noted during endoscopy. Local
recurrence was defined as recurrent cancer detected
at the primary resection site during follow-up
oesophagogastroduodenoscopy when pathological
review of the ESD specimen revealed no tumour on
the lateral and vertical margins.
Statistical analyses
Statistical analyses were done mainly using
descriptive analysis. The predictors of morbidity
and local recurrences were analysed by multivariate
logistic regression analysis using the following factors:
sex, American Society of Anesthesiologists grading,
presence of neoplastic lesions (adenoma, dysplasia,
carcinoma), ESD performed as a staging procedure,
the need for piecemeal resection, pathological size,
lateral and vertical margin involvement, depth
of pathological invasion, R0 resection, and the
institution volume of ESD. The 2-year overall and
disease-specific survivals were calculated using
the Kaplan-Meier estimator. A two-sided P value
of <0.05 was considered statistically significant.
Statistical analyses of data were performed using the
Statistical Package for the Social Sciences (Windows
version 20.0; SPSS Inc, Chicago [IL], US).
Results
During the study period, 187 lesions in 168 patients
were resected. The patient demographics are shown
in Table 1. The mean (± standard deviation) age of
patients was 61.9 ± 14.3 years and 61.3% were male.
Overall, EMR was performed in 34 (18.2%) lesions
and ESD in 153 (81.8%) lesions for dysplasia or
carcinoma with the majority of lesions located in
the stomach (132 lesions, 70.6%), followed by the
oesophagus (43 lesions, 23%) and the duodenum
(12 lesions, 6.4%). The distribution in the numbers
of the procedures among various hospitals is shown
in Figure a.
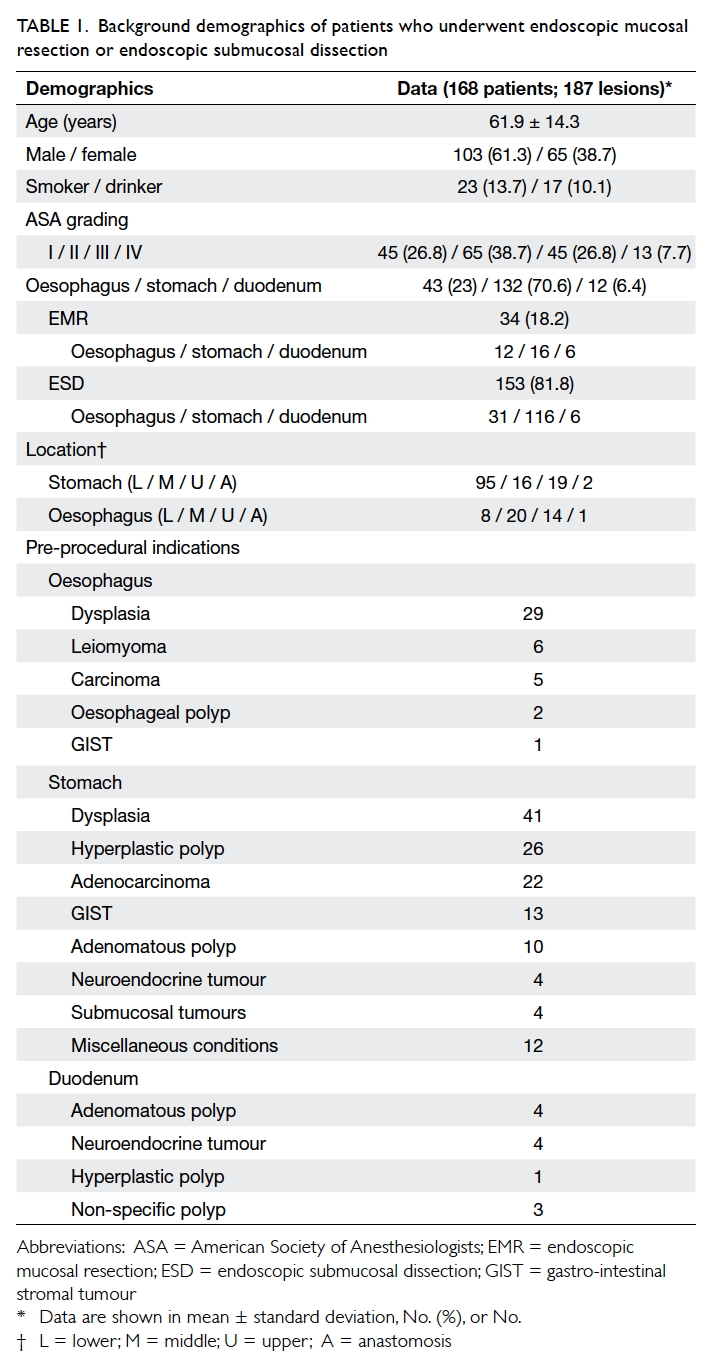
Table 1. Background demographics of patients who underwent endoscopic mucosal resection or endoscopic submucosal dissection
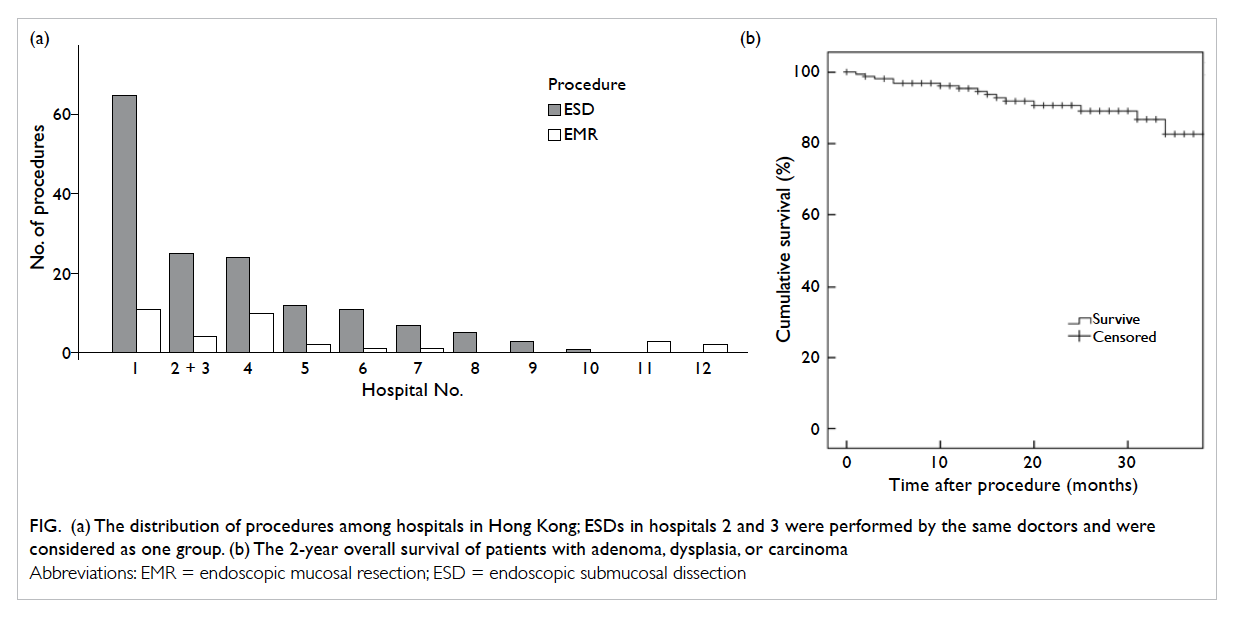
Figure. (a) The distribution of procedures among hospitals in Hong Kong; ESDs in hospitals 2 and 3 were performed by the same doctors and were considered as one group. (b) The 2-year overall survival of patients with adenoma, dysplasia, or carcinoma
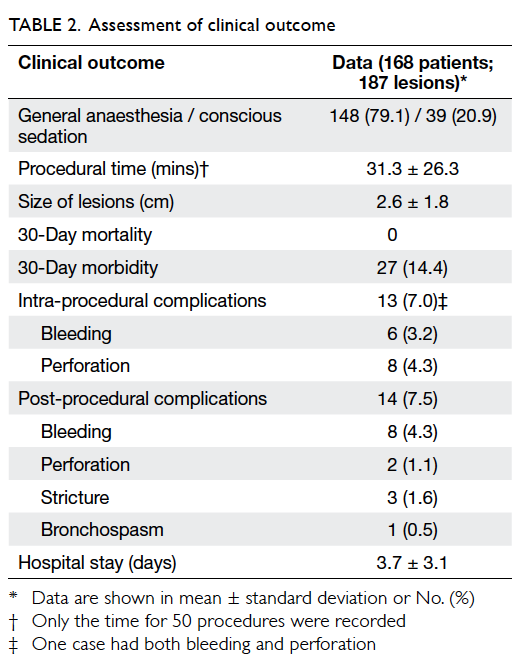
The clinical outcomes of the procedures
are shown in Table 2. General anaesthesia with tracheal intubation was required for the majority of
procedures (n=148, 79.1%). The procedural times
were recorded in 50 (26.7%) procedures only and
were not representative of the actual time required
for the procedures. The mean size of the lesions was
2.6 ± 1.8 cm. The 30-day morbidity rate was 14.4%.
Intra-procedural complications occurred in 13 (7.0%)
procedures, with one procedure having both
bleeding and perforation. Perforations occurred
in eight (4.3%) procedures and all were controlled
with endoscopic clipping. Severe bleeding requiring
endoscopic clipping or transfusion occurred in
six (3.2%) procedures. One procedure required
conversion to open surgery due to uncontrolled
bleeding. Post-procedural complications occurred
in 14 (7.5%) procedures; the most common causes
of which were bleeding (4.3%), stricture formation
(1.6%), and perforations (1.1%). All post-procedural
bleeding and stricture formation were managed
endoscopically. The procedures having perforation
were managed conservatively. No patients had post-procedural
mortality. The mean hospital stay was 3.7
± 3.1 days. Haemoglobin level was checked on the
first post-procedural day in 116 (62%) procedures.
Proton pump inhibitors were prescribed to 171
(91.4%) procedures; of these, 93 (80.2%) were
administered to ESD in the stomach. No difference
in size of lesions, hospital stay, or morbidities was
observed between university– and non-university–affiliated hospitals.
The types of knives used for mucosal incision
included the Dual knife (35.8%; KD-650U, Olympus
Co Ltd, Tokyo, Japan), the triangular tip knife (14.4%;
KD-640L, Olympus Co Ltd, Tokyo, Japan), the
needle knife (8%; KD-1L-1, Olympus Co Ltd, Tokyo,
Japan), the insulated tip (IT2) knife (7%; KD-610L,
Olympus Co Ltd, Tokyo, Japan), and the Hybrid
knife (3.7%; ERBE Tübingen, Germany). Knives used
for submucosal dissection included the Dual knife
(48%; KD-650U, Olympus Co Ltd), the insulated tip
(IT2) knife (24.6%; KD-610L, Olympus Co Ltd), the
triangular tip knife (11.2.%; KD-640L, Olympus Co
Ltd), and the Hybrid knife (4.3%; ERBE).
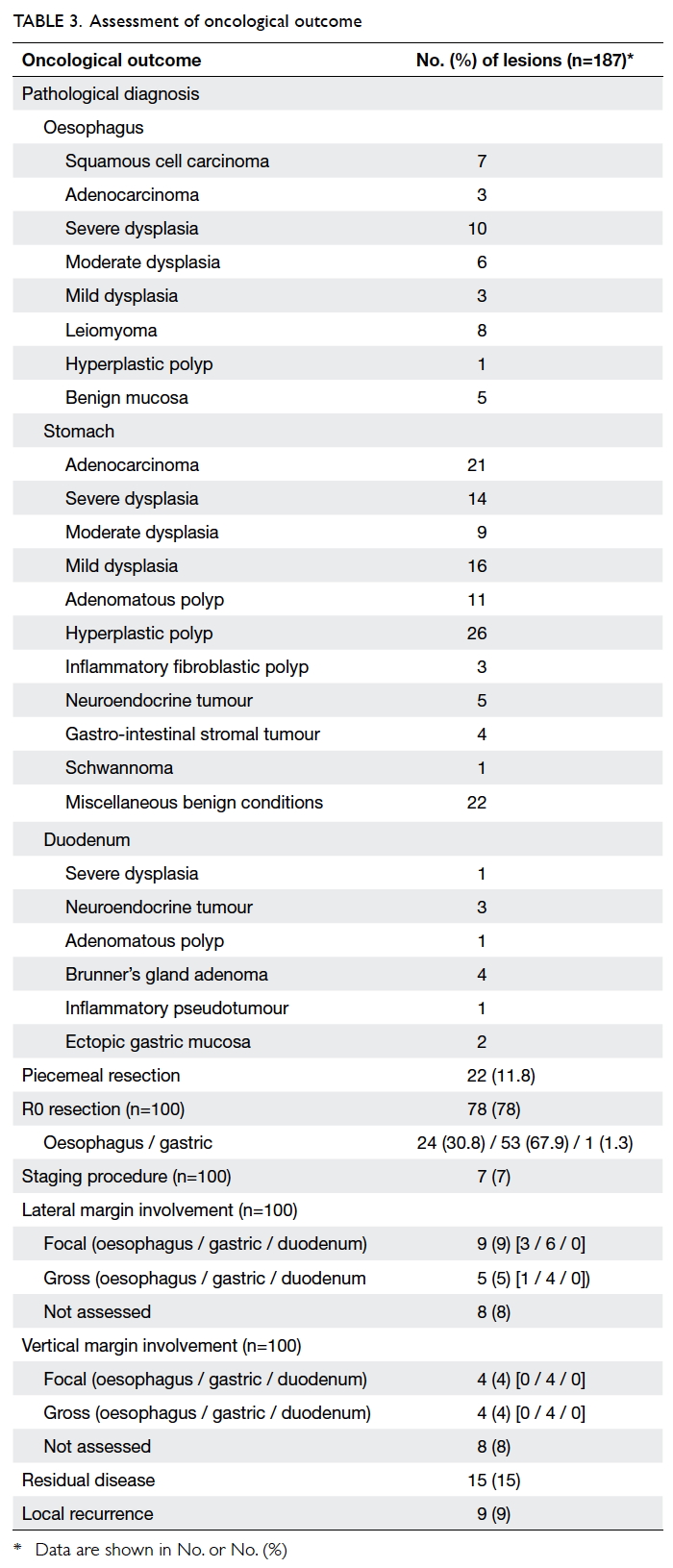
The oncological outcome of the procedures
is shown in Table 3. In 22 (11.8%) lesions, en-bloc
resection was not possible and the lesions had to
be resected in piecemeal. Among patients having
adenoma, dysplasia, or carcinoma (n=100), R0
resection was achieved in 78%. In 7% of the patients,
pre-procedural investigations were inconclusive on
the feasibility of endoscopic resection and ESD was
performed as a staging procedure. Lateral margin
involvement occurred in 14 (14%) procedures and
vertical margin involvement occurred in eight (8%)
procedures. Local recurrences occurred in nine
(9%) patients and five were treated by further ESD.
Residual disease was present in 15 (15%) patients,
four due to failure to complete ESD and 11 due
to margin involvement. Of these 15 patients with
residual disease, complete resection was achieved
in eight during salvage surgical resection, two
patients underwent repeat endoscopic resection,
two underwent radiofrequency ablation, and three
refused treatment. The mean follow-up time of
patients was 20.6 ± 10.8 months and the 2-year overall
survival rate and disease-specific survival rate was
90.6% and 100%, respectively (Fig b). Overall, 38.5%
of patients were followed up for at least 2 years. No
differences in oncological outcomes were observed
between university– and non-university–affiliated
hospitals.
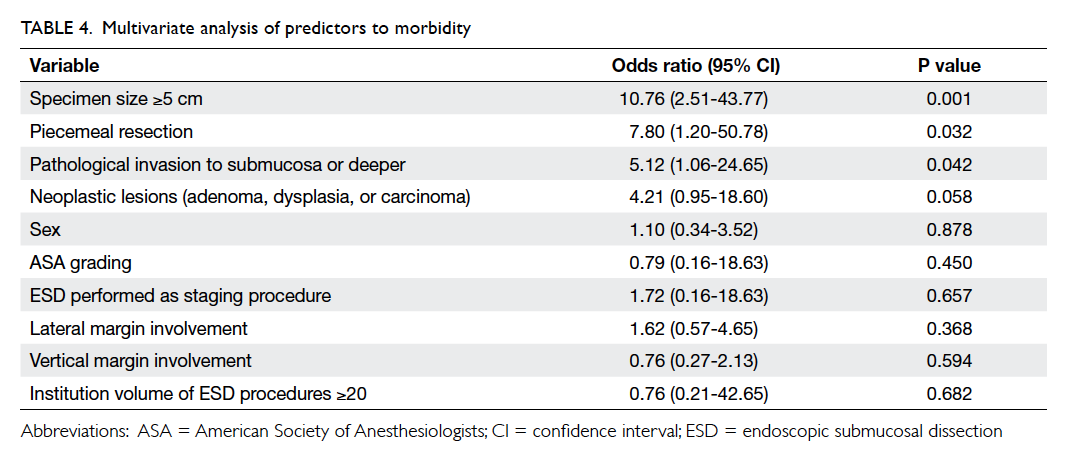
The predictors of morbidity in patients who
received endoscopic resection were then analysed
with multivariate logistic regression (Table
4). Specimen size of ≥5 cm (P=0.001), the need for
piecemeal resection (P=0.032), and pathological
invasion to the submucosa or deeper (P=0.042) were
independent predictors of morbidity. The predictors
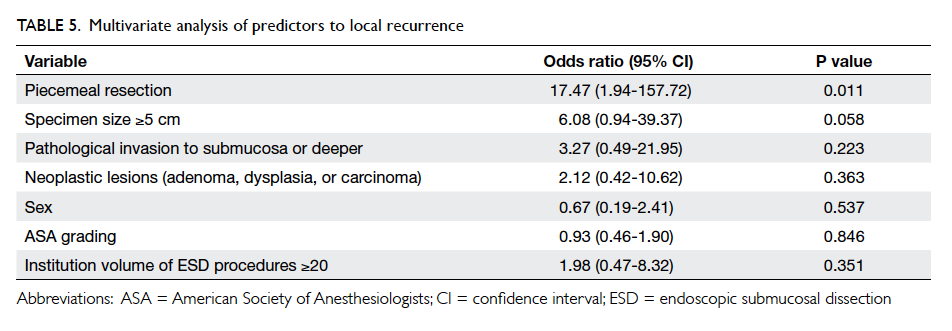
for local recurrences were also analysed: the need
for piecemeal resection was the only independent
predictor (P=0.011; Table 5).
Discussion
Using a hospital admission database, the outcome
for patients who underwent endoscopic resection of
superficial upper gastro-intestinal neoplasms among
12 government-funded hospitals in Hong Kong was
reviewed. The total number of procedures performed
was higher than expected and the procedures were
associated with a low risk of morbidity. Nonetheless
there may be room for improvement in the
oncological outcome, particularly in terms of rates
of margin involvement and the number of patients
with residual disease. The predictors of morbidity
and local recurrence were in line with those reported
from high-volume centres and the 2-year survival of
patients who had adenoma, dysplasia, or carcinoma
was excellent following endoscopic resection.
The adoption of ESD outside Japan and
Korea has been slow.5 8 15 16 17 18 The reported studies were
mostly small series and the results were variable. In
four European and two South-East Asian studies, the
number of patients included was between 23 and 70,
the en-bloc resection rate ranged from 25% to 100%
and morbidity 0% to 24%. These results contrast
with those obtained from Japan and Korea. In the
stomach, the rate of en-bloc resection was 94.9%
to 95.3% and piecemeal resection was 4.1%. The
risk of bleeding was 1.8% to 16.2% and perforation
was 1.2% to 4.5%. The risk of recurrence was less
than 1% and the 3- and 5-year overall survival rates
were 98.4% and 97.1% respectively.6 7 These results reflect the difficulty of introducing a technically
demanding ESD programme in low-to-medium volume localities outside Japan and Korea.19 The reasons for poorer clinical and oncological outcomes
may be explained by a combination of factors. These
include the early experience and learning curve
issues, technical difficulties leading to the need for
piecemeal resection, and failure to recognise tumour
margins.
The current study is the largest non-Japanese
and non-Korean report on the outcomes of ESD. The
results illustrate that an acceptable en-bloc resection
rate and morbidity rate can be achieved in low-to-medium–volume hospitals. The introduction of
endoscopic resection techniques in Hong Kong is
in its infancy with many challenges similar to those
described in western literature.20 21 22 The emphasis in
our region, however, was not just on the technical
performance of ESD but also the ability to detect
and diagnose these lesions. In 2011, a total of 1101
new cases of gastric cancer were diagnosed in Hong
Kong but the percentage of cases that were amenable
to endoscopic resection is unknown.23 Based on the
findings of this study, the percentages are likely to
have been much less than 10%. This figure contrasts
significantly with those reported from Spain (20%)
and Japan (53%).24 25 Hence, measures to further improve the early diagnosis of upper gastro-intestinal
malignancies are needed. Key personnel
to perform ESD in individual hospitals should be
identified and receive intensive training in screening
endoscopy to improve detection of early lesions.26
These individuals should attend local workshops
and clinical attachments at high-volume centres in
Japan and Korea to acquire such skills. Enhanced
endoscopic imaging systems (narrow band imaging,
flexible spectral imaging colour enhancement, or
autofluorescence imaging) that may improve the ease
of diagnosing early upper gastro-intestinal lesions
should also be obtained by those hospitals interested
in developing the technique and these should be
supported by the government on a regional scale.27 28 29
The use of population-based screening programmes
in our locality may not be justified because of the
moderate incidence of upper gastro-intestinal
cancers, but studies to evaluate screening of high-risk
groups may be justified.30 31 Better recognition of early upper gastro-intestinal neoplasms not only
increases the detection rates but may also allow more
accurate pre-ESD assessment and improve the rates
of margin involvement and oncological outcomes.
It is acknowledged that ESD remains a
technically demanding procedure that is associated
with risk of perforation and bleeding. To master such
skills, the surgeon must be familiar not only with
the ESD procedure, but also with the methods used
to treat complications. In addition, the difficulty of
ESD depends on the organ involved, as well as the
location within that organ.32 33 Thus, in high-volume centres, learning of ESD is usually done in a stepwise
approach, starting from easy small lesions located at
the antrum of the stomach and progressing to more
difficult lesions or lesions located in other parts of
the gastro-intestinal tract.34 35 The number required for mastering the technique is 20 to 40 procedures
in each location. These numbers are unlikely to be
attainable within a short period of time in Hong
Kong and most western countries. Hence, each
locality will need to decide on the most appropriate
training strategy to overcome the learning curve:
this will likely involve intensive training with the use
of animal models, apprenticeship with local experts,
and overseas training in high-volume centres.13 20 24
It may also be beneficial to the region to restrict
the performance of ESD to a few key personnel in
the main institutions and once they are proficient,
involve other endoscopists in the process.
To further improve the outcome of endoscopic
resection of upper gastro-intestinal lesions in
Hong Kong, a number of measures should be
implemented. The establishment of a task force on
endoscopic diagnosis and treatment of early gastro-intestinal
cancers with regular training should
be considered. A local standard in endoscopic
reporting and pathological examination is required
as these assessments of early upper gastro-intestinal
neoplasms are unique and pivotal to guiding
treatment. A ‘best practice’ guideline for performing
ESD and the provision of pre- and post-ESD care
should be established to provide a benchmark for
future audits.
There were a number of limitations to the
current study. First, patients included in the study
were identified using CDARS based on the diagnosis
and procedural coding. There is a risk of unidentified
procedures if they were incorrectly coded. Also,
those procedures that were performed outside the
Hospital Authority hospitals could not be accounted
for. Second, since this is a retrospective review, some
outcome parameters might not have been properly
defined or available for all patients. Finally, since
there is no standardised reporting system in Hong
Kong for ESD or EMR and pathological assessment,
there was a risk of information bias during extraction
of the data.
Conclusion
The introduction of ESD in Hong Kong is still in
its infancy; EMR and ESD were introduced in low-to-moderate–volume hospitals with acceptable
morbidity rates. The short-term survival was
excellent. Nonetheless, other oncological outcomes
were higher than those observed in high-volume
centres and more secondary procedures were
required.
References
1. Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic
mucosal resection for early cancers of the upper
gastrointestinal tract. J Clin Oncol 2005;23:4490-8. Crossref
2. Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic
submucosal dissection compared with EMR for early
gastric cancer. Gastrointest Endosc 2006;64:877-83. Crossref
3. Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic
submucosal dissection of esophageal squamous cell
neoplasms. Clin Gastroenterol Hepatol 2006;4:688-94. Crossref
4. Fujishiro M, Yahagi N, Kakushima N, et al. Outcomes of
endoscopic submucosal dissection for colorectal epithelial
neoplasms in 200 consecutive cases. Clin Gastroenterol
Hepatol 2007;5:678-83. Crossref
5. Chiu PW, Chan KF, Lee YT, Sung JJ, Lau JY, Ng EK.
Endoscopic submucosal dissection used for treating early
neoplasia of the foregut using a combination of knives.
Surg Endosc 2008;22:777-83. Crossref
6. Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic
submucosal dissection for early gastric cancer: a large-scale
feasibility study. Gut 2009;58:331-6. Crossref
7. Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in
1000 cases of endoscopic submucosal dissection for early
gastric neoplasms: Korean ESD Study Group multicenter
study. Gastrointest Endosc 2009;69:1228-35. Crossref
8. Probst A, Golger D, Arnholdt H, Messmann H. Endoscopic
submucosal dissection of early cancers, flat adenomas,
and submucosal tumors in the gastrointestinal tract. Clin
Gastroenterol Hepatol 2009;7:149-55. Crossref
9. Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa
N, Lopes C, Moreira-Dias L. A European case series of
endoscopic submucosal dissection for gastric superficial
lesions. Gastrointest Endosc 2009;69:350-5. Crossref
10. Repici A, Hassan C, Carlino A, et al. Endoscopic
submucosal dissection in patients with early esophageal
squamous cell carcinoma: results from a prospective
Western series. Gastrointest Endosc 2010;71:715-21. Crossref
11. Probst A, Golger D, Anthuber M, Märkl B, Messmann H.
Endoscopic submucosal dissection in large sessile lesions
of the rectosigmoid: learning curve in a European center.
Endoscopy 2012;44:660-7. Crossref
12. Kakushima N, Fujishiro M, Kodashima S, Muraki Y,
Tateishi A, Omata M. A learning curve for endoscopic
submucosal dissection of gastric epithelial neoplasms.
Endoscopy 2006;38:991-5. Crossref
13. Teoh AY, Chiu PW, Wong SK, Sung JJ, Lau JY, Ng
EK. Difficulties and outcomes in starting endoscopic
submucosal dissection. Surg Endosc 2010;24:1049-54. Crossref
14. Berr F, Ponchon T, Neureiter D, et al. Experimental
endoscopic submucosal dissection training in a porcine
model: learning experience of skilled Western endoscopists.
Dig Endosc 2011;23:281-9. Crossref
15. Rösch T, Sarbia M, Schumacher B, et al. Attempted
endoscopic en bloc resection of mucosal and submucosal
tumors using insulated-tip knives: a pilot series. Endoscopy
2004;36:788-801. Crossref
16. Farhat S, Chaussade S, Ponchon T, et al. Endoscopic
submucosal dissection in a European setting. A multi-institutional
report of a technique in development.
Endoscopy 2011;43:664-70. Crossref
17. Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle
M, Neuhaus H. Endoscopic submucosal dissection of
early gastric neoplasia with a water jet-assisted knife: a
Western, single-center experience. Gastrointest Endosc
2012;75:1166-74. Crossref
18. Chang CC, Lee IL, Chen PJ, et al. Endoscopic submucosal
dissection for gastric epithelial tumors: a multicenter study
in Taiwan. J Formos Med Assoc 2009;108:38-44. Crossref
19. Chiu PW. Novel endoscopic therapeutics for early gastric
cancer. Clin Gastroenterol Hepatol 2014;12:120-5. Crossref
20. Coman RM, Gotoda T, Draganov PV. Training in
endoscopic submucosal dissection. World J Gastrointest
Endosc 2013;5:369-78. Crossref
21. Neuhaus H. Endoscopic submucosal dissection in the
upper gastrointestinal tract: present and future view of
Europe. Dig Endosc 2009;21 Suppl 1:S4-6. Crossref
22. Deprez PH, Bergman JJ, Meisner S, et al. Current practice
with endoscopic submucosal dissection in Europe: position
statement from a panel of experts. Endoscopy 2010;42:853-8. Crossref
23. Hong Kong Cancer Registry, Hospital Authority. Available
from: http://www3.ha.org.hk/cancereg/. Accessed 11 Feb
2013.
24. Miguélez Ferreiro S, Cornide Santos M, Martínez Moreno
E. Gastric cancer in a Spanish hospital: Segovia General
Hospital (2005-2008) [in Spanish]. Gastroenterol Hepatol
2012;35:684-90. Crossref
25. Sugano K. Gastric cancer: pathogenesis, screening, and
treatment. Gastrointest Endosc Clin N Am 2008;18:513-22, ix. Crossref
26. Yamazato T, Oyama T, Yoshida T, et al. Two years’ intensive
training in endoscopic diagnosis facilitates detection of
early gastric cancer. Intern Med 2012;51:1461-5. Crossref
27. Muto M, Minashi K, Yano T, et al. Early detection of
superficial squamous cell carcinoma in the head and neck
region and esophagus by narrow band imaging: a multicenter
randomized controlled trial. J Clin Oncol 2010;28:1566-72. Crossref
28. Jung SW, Lim KS, Lim JU, et al. Flexible spectral imaging
color enhancement (FICE) is useful to discriminate among
non-neoplastic lesion, adenoma, and cancer of stomach.
Dig Dis Sci 2011;56:2879-86. Crossref
29. Lee JH, Cho JY, Choi MG, et al. Usefulness of
autofluorescence imaging for estimating the extent of
gastric neoplastic lesions: a prospective multicenter study.
Gut Liver 2008;2:174-9. Crossref
30. Takenaka R, Kawahara Y, Okada H, et al. Narrow-band
imaging provides reliable screening for esophageal
malignancy in patients with head and neck cancers. Am J
Gastroenterol 2009;104:2942-8. Crossref
31. Leung WK, Wu MS, Kakugawa Y, et al. Screening for
gastric cancer in Asia: current evidence and practice.
Lancet Oncol 2008;9:279-87. Crossref
32. Oda I, Odagaki T, Suzuki H, Nonaka S, Yoshinaga S.
Learning curve for endoscopic submucosal dissection
of early gastric cancer based on trainee experience. Dig
Endosc 2012;24 Suppl 1:129-32. Crossref
33. Murata A, Okamoto K, Muramatsu K, Matsuda S.
Endoscopic submucosal dissection for gastric cancer: the
influence of hospital volume on complications and length
of stay. Surg Endosc 2014;28:1298-306. Crossref
34. Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, Bae JM.
The learning curve for EMR with circumferential mucosal
incision in treating intramucosal gastric neoplasm.
Gastrointest Endosc 2005;62:860-5. Crossref
35. Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning
curve for advanced endoscopic resection. Gastrointest
Endosc 2005;62:866-7. Crossref