Hong Kong Med J 2015 Apr;21(2):149–54 | Epub 5 Dec 2014
DOI: 10.12809/hkmj144330
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Risks and benefits of citrate anticoagulation for continuous renal replacement therapy
HP Shum, FHKCP, FHKAM (Medicine)1; WW Yan, FHKCP, FHKAM (Medicine)1; TM Chan, MD, FHKAM (Medicine)2
1 Department of Intensive Care, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr HP Shum (shumhp@ha.org.hk)
Abstract
Heparin, despite its significant side-effects, is the
most commonly used anticoagulant for continuous
renal replacement therapy in critical care setting.
In recent years, citrate has gained much popularity
by improving continuous renal replacement therapy
circuit survival and decreasing blood transfusion
requirements. However, its complex metabolic
consequences warrant modification in the design
of the citrate-based continuous renal replacement
therapy protocol. With thorough understanding
of the therapeutic mechanism of citrate, a simple
and practicable protocol can be devised. Citrate-based
continuous renal replacement therapy can be safely and widely used in the clinical setting with
appropriate clinical staff training.
Introduction
Continuous renal replacement therapy (CRRT) is
commonly used for the management of acute kidney
injury in the intensive care unit (ICU) worldwide.
Anticoagulation is necessary to prevent clotting of
the extracorporeal circuit. Unfractionated heparin
has the advantages of acceptable circuit life, low cost,
easy monitoring and simple reversal, and hence,
remains a popular choice.1 2 However, critically ill patients have various bleeding risks that may contra-indicate
the use of heparin. The contra-indications
may include recent surgical procedures, multiple
trauma, thrombocytopenia, and coagulation
defects.3 Moreover, binding of heparin to endothelial
antithrombin inhibits its anti-inflammatory actions
and prevents local prostacyclin formation that,
in turn, jeopardises micro-circulation.4 5 Hence, heparin use may be disadvantageous in patients with
Gram-negative sepsis.4 Although implementation
of an anticoagulation-free regimen is a safer
alternative, circuit clotting occurs frequently, which
not only increases treatment cost and downtime, but
also increases the need of blood transfusion and the
nursing workload. Among the various alternative
anticoagulants like low-molecular-weight heparin,
serine proteinase inhibitor nafamostat, prostacyclin,
hirudin and direct thrombin inhibitor, citrate
has gained popularity in recent years. This paper
provides a general review on citrate use in CRRT and
focuses on studies published in the past decade.
Mechanism of action
Citrate just mixes with the blood before it enters the CRRT circuit as illustrated in the commonly
used citrate-based CRRT regimens (Figures 1 to 3). Citrate chelates the ionised calcium, which
is essential for the normal coagulation cascade
and results in inhibition of thrombin generation.
An extracorporeal, post-filter, ionised calcium
concentration of 0.25 to 0.35 mmol/L is effective to
achieve the anticoagulation effect. The majority of
citrate is removed by either filtration or dialysis with
a sieving coefficient of one in both processes.6 The
removal fraction varies from 20% to 80%, depending
on the blood flow rate, effluent flow rate, and CRRT
modality.7 8 The remaining calcium-citrate product enters the systemic circulation and is metabolised
in the liver, muscles, and kidneys to produce three
molecules of bicarbonate for every molecule of
citrate. Replacement infusion of calcium is commonly
given to compensate the extracorporeal loss and to
normalise a patient’s systemic calcium level.8 9 The relative contra-indications for citrate-based CRRT
include liver failure with or without cirrhosis, severe
hypoxaemia, and after massive blood transfusion.
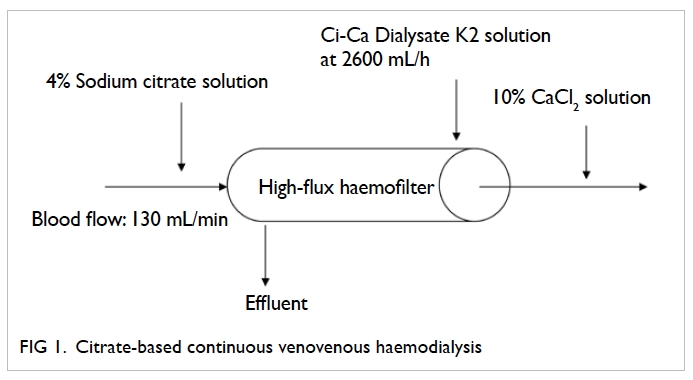
Figure 1. Citrate-based continuous venovenous haemodialysis
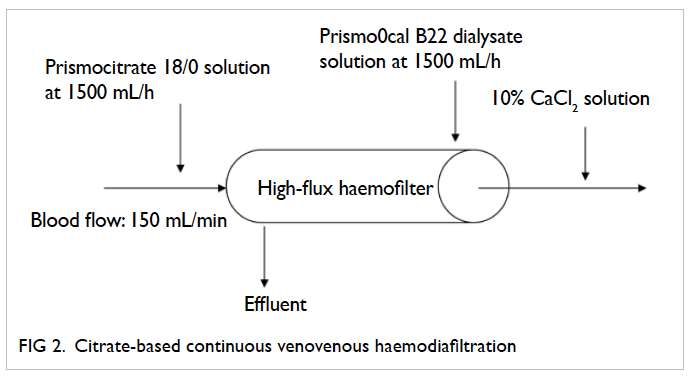
Figure 2. Citrate-based continuous venovenous haemodiafiltration
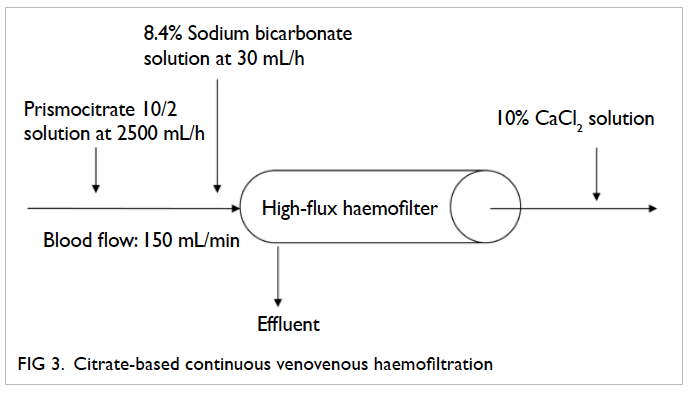
Figure 3. Citrate-based continuous venovenous haemofiltration
Circuit survival
Factors affecting the circuit life include a patient’s
clinical condition and coagulation status, the position
and patency of the vascular access, the choice of
anticoagulant, modality of CRRT, and filtration
fraction.10 11 Most of the published randomised controlled trials (RCTs) indicate improved circuit
survival with citrate versus heparin (Table 112 13 14 15 16 17).
Three meta-analyses have been published recently
that summarise this issue.18 19 20 While Liao et al20
only focused on RCTs that compare unfractionated heparin with citrate, Zhang and Hongying18 and Wu et al19 also included studies on low-molecular-weight
heparin and regional heparin. They concluded that circuit life with citrate was comparable19 20 or better
(by a mean difference of 23.03 hours; 95% confidence interval, 0.45-45.61 hours) than that with heparin.18
The main reason for the discrepancy between the
findings by Zhang and Hongying18 (which showed
more favourable circuit life for citrate group vs heparin group) and Wu et al19 (which showed comparable circuit life between citrate and heparin
groups) was attributed to the study by Betjes et al,17
which did not report interquartile ranges of circuit survival. While Zhang and Hongying18 estimated the survival time in this study by Kaplan-Meier analysis,
Wu et al19 excluded it from their circuit survival
analysis. With the improved circuit life, citrate can
decrease circuit downtime and minimise discrepancy
between prescribed and delivered CRRT dose,
achieve lower treatment cost, avoid unnecessary
blood loss, and reduce nursing workload. In fact, the
latest Kidney Disease Improving Global Outcomes
Clinical Practice Guidelines recommended citrate as
the anticoagulant of choice in patients requiring
CRRT.21
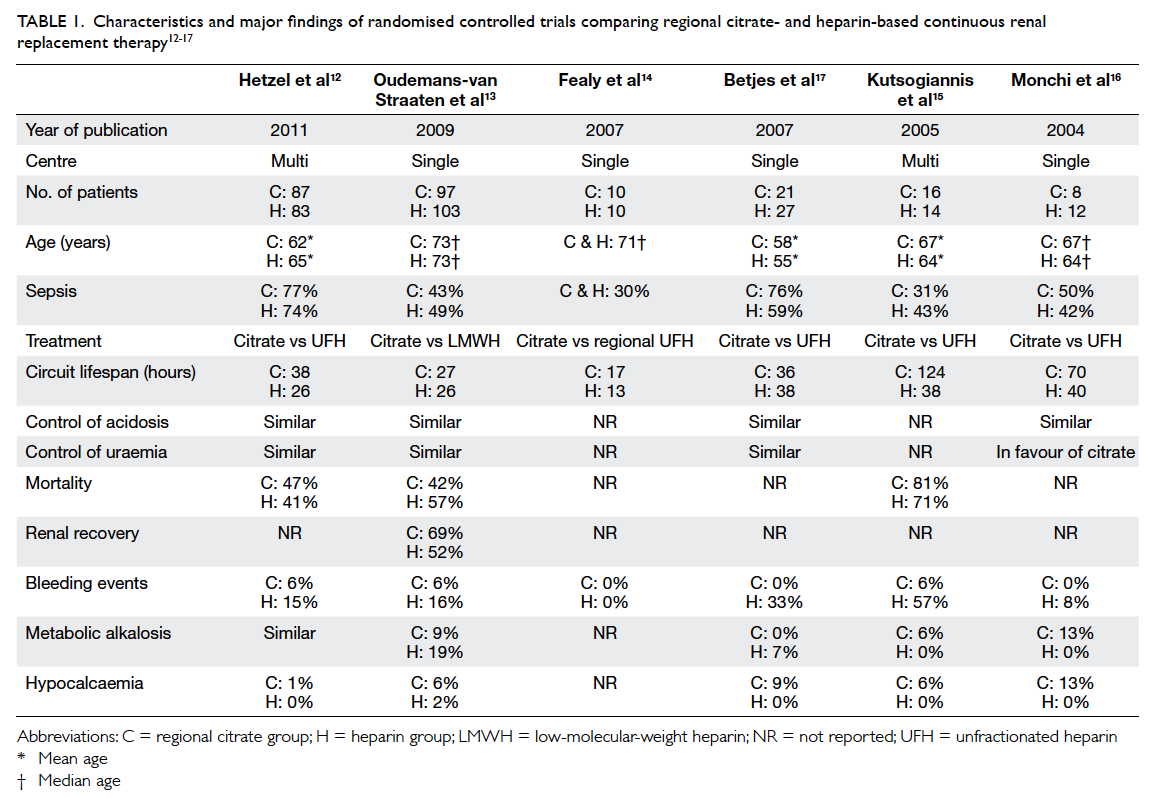
Table 1. Characteristics and major findings of randomised controlled trials comparing regional citrate- and heparin-based continuous renal replacement therapy
Metabolic control
Concerning reversal of metabolic acidosis and
control of uraemia, most RCTs12 13 17 reported similar
efficacy when comparing citrate- with heparin-based
CRRT, except one study16 which was in favour
of citrate. This might be explained by the longer
circuit lifetime, which offered better uraemic toxin
clearance.
Bleeding events
All RCTs,12 13 15 16 17 except one,14 showed a higher
bleeding risk with heparin-based CRRT when
compared with citrate anticoagulation (Table 1).
All the three meta-analyses18 19 20 demonstrated a
significantly lower incidence of bleeding in citrate-based
CRRT compared with heparin, with a pooled
risk ratio ranging from 0.25 to 0.34. However,
the definition of bleeding events varied in all the
included trials. Blood transfusion requirement was
lower in the citrate group when compared with the
unfractionated heparin group.20
Mortality
Three studies provided outcome information on
mortality (Table 1).12 13 15 The study by Kutsogiannis
et al15 was relatively small (n=30, mortality rate of
citrate group vs heparin group = 81% vs 71%; P=0.69)
and was not powered to detect survival difference.
The single-centre study by Oudemans-van Straaten
et al13 showed a mortality benefit at 3 months (48%
vs 63% respectively; P=0.03) when comparing citrate
with low-molecular-weight heparin. Mortality was
mainly reduced in surgical patients, those with
sepsis, those younger (<73 years), and in patients
with higher Sepsis-related Organ Failure Assessment
scores (>11 points). The study speculated that
citrate, apart from being an excellent anticoagulant
for CRRT, might have immunomodulatory actions
that suppress inflammation, thus, leading to a
survival benefit in critically ill patients.13 However,
a subsequent multicentre study by Hetzel et al12
failed to demonstrate such benefit. While Hetzel
et al12 used unfractionated heparin, Oudemans-van
Straaten et al13 used a low-molecular-weight heparin
preparation. Hetzel et al12 also included subjects
who were younger and more septic. The discrepancy
in clinical background and the different mode of
anticoagulation might explain the difference in the
observed results. It was important to note that both
studies were not sufficiently powered to ascertain
survival benefit of regional citrate anticoagulation,
and mortality rate was not the primary end-point. A
properly powered, well-designed RCT is required to
clarify this issue.
Side-effects
Citrate anticoagulation has complex metabolic
consequences due to its physiochemical property.
Apart from being an anticoagulant, it also serves as
a pH buffer, chelating agent, and a source of energy
and sodium.
Citrate toxicity
A recent pharmacokinetic study showed that citrate
clearance is not impaired in critically ill patients.22
However, reduced metabolism of citrate occurs
in patients with chronic liver disease, ischaemic
hepatitis, hypoxia and impaired muscle perfusion,
which are commonly found in the ICU setting. Citrate
accumulation results in ionised hypocalcaemia and
acidosis, which cause hypotension due to decreased
myocardial contractility and vascular hypotonia. A
paradoxical increase in total calcium concentration
often occurs due to increased citrate-bound calcium,
as well as the increased use of calcium replacement
in response to ionised hypocalcaemia. The total-to-ionised calcium ratio is, therefore, an important
marker to detect citrate accumulation.23 24 An elevated ratio of >2.25 should alert the clinician to the possibility
of citrate accumulation. Impaired citrate metabolism
is an independent risk factor for mortality. A ratio of
≥2.4 independently predicted a 33.5-fold increase in
28-day mortality rate in critically ill patients.25 There
was also a significant correlation between total-to-ionised calcium ratio and the severity of critical
illness. Citrate toxicity should be suspected, based
on the presence of the following three observations,
namely, elevated total-to-ionised calcium ratio of
>2.25, increased use of calcium replacement, and
increasing metabolic acidosis. Clinically, patients
may present with symptoms of hypocalcaemia
like circumoral paraesthesia, carpopedal spasm,
generalised tetany, and hyper-reflexia.26 Prolonged QT
interval may follow by the development of Torsades
de pointes or ventricular arrhythmia in untreated
patients.26 Confirmation of citrate intoxication can
only be done by checking citrate concentration in
blood, which is not readily available in most hospital
laboratories. Therefore, clinical symptoms and signs
are suggestive but not diagnostic of citrate toxicity.
In highly suspicious cases, citrate infusion should
be stopped immediately, followed by intravenous
calcium injection. Continuous renal replacement
therapy could be resumed using citrate-free regimen
after initial stabilisation. Despite the potential risks
associated with citrate toxicity, CRRT with citrate
anticoagulation is considered feasible in patients with
liver impairment,27 provided that careful monitoring
of calcium level and meticulous titration are ensured.
Citrate accumulation should be minimised with the
reduction in citrate infusion, increase in effluent
flow to promote citrate clearance, adequate calcium
replacement to counteract hypocalcaemia, and
supplementation with extra bicarbonate to correct
acidosis.
Metabolic derangement
Recent meta-analyses showed no significant
difference in the incidence of metabolic alkalosis
in citrate groups compared with heparin
groups in patients with normal metabolism.19 20 Hypernatraemia is a problem commonly associated
with the use of concentrated citrate solution (4%
trisodium citrate solution has 544 mmol/L sodium
while Anticoagulant Citrate Dextrose Solution A [ACDA] has 224 mmol/L sodium). Adoption of a
slightly hyponatraemic replacement or dialysate
solution in the CRRT regimen may be a remedy to
this problem. Alternatively, normonatraemic citrate
solution may be used, for instance, Prismocitrate 18/0
or Prismocitrate 10/2 (Gambro Hospal, Stockholm,
Sweden), which serves as both an anticoagulant and
a source of buffer for predilutional treatment.28 29 The
drawback of this method is that it does not guarantee
a fixed relationship between citrate and blood flow.
This is due to the fact that the amount of replacement
fluid entering the circuit is dependent on filtrate
flow and the desired amount of fluid removal,
which are prone to variations. The varying citrate
concentration may consequently exert a negative
effect on the circuit survival time. This issue can
be resolved by fixing the flow ratio between blood
and the citrate-containing substitution fluid. Citrate
binds to magnesium, resulting in excessive loss in
filtrate and causing hypomagnesaemia, which in
turn decreases the release of parathyroid hormone,
promotes hypokalaemia, and induces tetany as well
as cardiac arrhythmia. Monitoring and replacement
of magnesium should be done regularly.
Energy gain
Citrate also serves as a source of energy with 0.59
kcal/mmoL and can enter cells without insulin.
The bioenergetic gain of citrate-anticoagulated
CRRT is not limited to citrate itself, but is also
contributed by glucose (in ACDA) and
lactate (in replacement or dialysate solution). The
energy delivered can differ substantially between
modalities, even with comparable doses.30 Such
information should, therefore, be taken into account
when nutritional needs are being calculated.
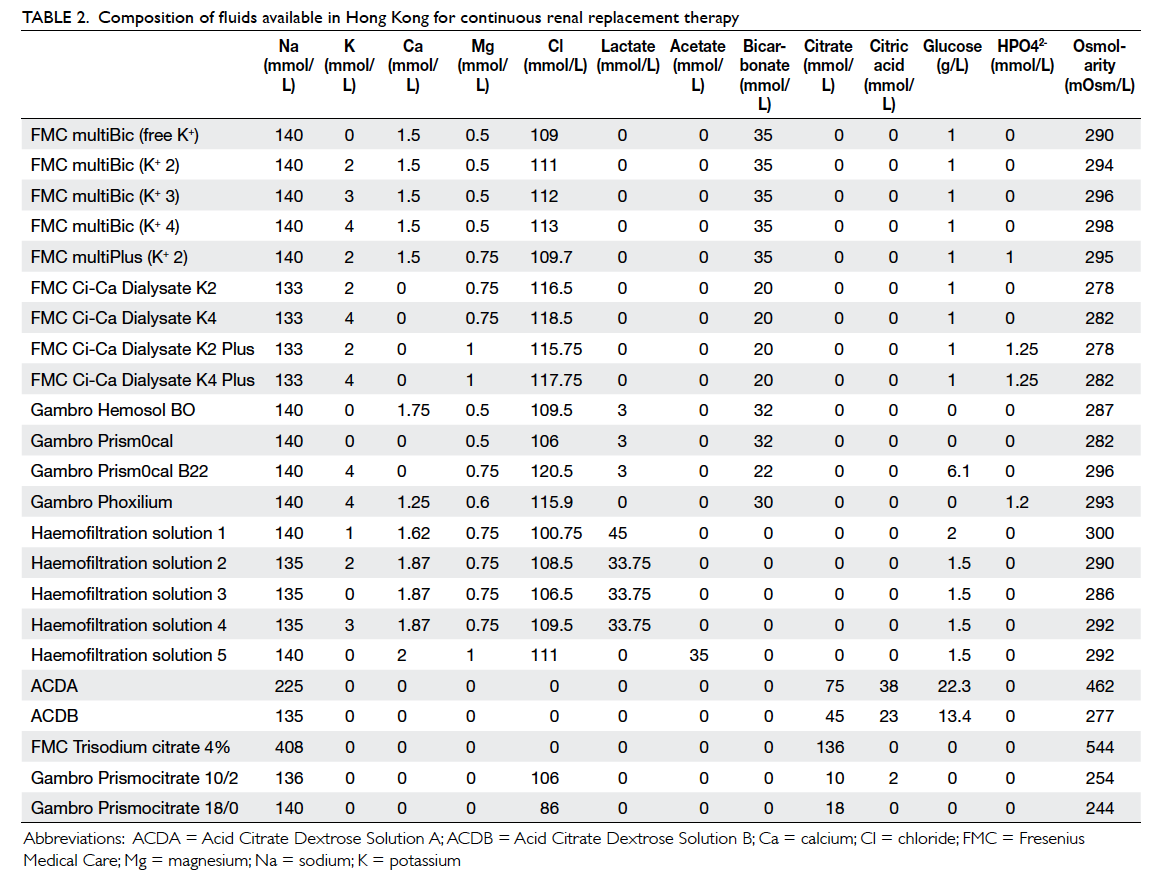
Treatment cost
The composition of commonly used fluid for CRRT
is shown in Table 2. Using average filter life (citrate
vs heparin = 52 hours vs 30 hours) calculated based
on previously published RCTs (Table 1), the total
treatment cost for citrate-based CRRT lasting for
72 hours is around HK$10 000 (using continuous
venovenous haemodiafiltration with 1250 mL/h pre-filter citrate containing replacement solution
plus 1250 mL/h dialysate flow rate and two
haemofilter/circuit changes within 72 hours of
treatment). The cost is similar to that of heparin-based
regimen (using continuous venovenous
haemodiafiltration with 1250 mL/h post-filter
replacement solution plus 1250 mL/h dialysate
flow rate and three haemofilter/circuit changes
within 72 hours of treatment).
Future development
Development of fluid for citrate-based continuous renal replacement therapy
The setup of regional citrate anticoagulant with
conventional CRRT machines was more complicated
compared with other anticoagulants. Since 1995,
citrate in the form of ACDA had been the default
anticoagulation method for CRRT in our unit, as
described by Leung and Yan.31 The solutions for
replacement are customised (with low bicarbonate
and sodium level) as a concentrated citrate solution
will provide extra sodium and bicarbonate load.
Reconstitution of this customised replacement fluid
was tedious, time-consuming, and error-prone. The
next evolution was to use online replacement fluid
generated from Gambro AK200 Ultra S (Gambro Hospal, Stockholm, Sweden) together with post-dilution
continuous venovenous haemofiltration
with citrate anticoagulation using ACDA.
The advantage of this system was the flexibility of
adjusting sodium and bicarbonate concentrations
with the replacement solution generated online. It
also came at a lower treatment cost due to generation
of sterile replacement fluid via the online system.32
However, generation of ultra-pure water is a prerequisite
for implementation, and ICUs without
pre-installed water delivery and treatment systems
will be precluded from this treatment technology. In
the recent 10 years, commercially prepared citrate-containing
replacement solution and tailor-made
dialysate have become widely available (Table 2),
and citrate-based CRRT has been widely adopted in
different ICUs.
Machine and protocol development
Previous CRRT machines were not specifically
designed for citrate-based treatment. Additional
infusion pumps for continuous citrate administration
were required during treatment, and posed major
safety problems. When the CRRT machine alarm
sounds for bag exchange or for other mechanical
problems, all CRRT infusion pumps will stop
except for the pump used to infuse the citrate
solution, which may result in the direct infusion
of citrate solution into the patient. Nowadays, the
new CRRT machines have incorporated integrated
citrate modules and specific protocols. Continuous
monitoring of citrate and ionised calcium levels
together with computerised algorithms may further
improve patient safety and minimise potential side-effects.
33 34 Yet, successful implementation of the
protocol requires focused and continuous training
for the involved clinical staff.
Conclusion
Citrate is a safe and effective anticoagulant for
CRRT. Its advantages can be fully appreciated with
a simple, well-devised and practicable protocol, and
appropriate clinical staff training.
References
1. Uchino S, Bellomo R, Morimatsu H, et al. Continuous
renal replacement therapy: a worldwide practice survey.
The beginning and ending supportive therapy for the
kidney (B.E.S.T. kidney) investigators. Intensive Care Med
2007;33:1563-70. Crossref
2. Davies H, Leslie G. Anticoagulation in CRRT: agents and
strategies in Australian ICUs. Aust Crit Care 2007;20:15-26. Crossref
3. van de Wetering J, Westendorp RG, van der Hoeven JG,
Stolk B, Feuth JD, Chang PC. Heparin use in continuous
renal replacement procedures: the struggle between filter
coagulation and patient hemorrhage. J Am Soc Nephrol
1996;7:145-50.
4. Hoffmann JN, Vollmar B, Laschke MW, et al. Adverse effect
of heparin on antithrombin action during endotoxemia:
microhemodynamic and cellular mechanisms. Thromb
Haemost 2002;88:242-52.
5. Oudemans-van Straaten HM, Kellum JA, Bellomo R.
Clinical review: anticoagulation for continuous renal
replacement therapy—heparin or citrate? Crit Care
2011;15:202. Crossref
6. Chadha V, Garg U, Warady BA, Alon US. Citrate clearance
in children receiving continuous venovenous renal
replacement therapy. Pediatr Nephrol 2002;17:819-24. Crossref
7. Hartmann J, Strobl K, Fichtinger U, Schildböck C,
Falkenhagen D. In vitro investigations of citrate clearance
with different dialysis filters. Int J Artif Organs 2012;35:352-9. Crossref
8. Oudemans-van Straaten HM, Ostermann M. Bench-to-bedside
review: Citrate for continuous renal replacement
therapy, from science to practice. Crit Care 2012;16:249. Crossref
9. Tolwani A, Wille KM. Advances in continuous renal
replacement therapy: citrate anticoagulation update. Blood
Purif 2012;34:88-93. Crossref
10. Joannidis M, Oudemans-van Straaten HM. Clinical review:
Patency of the circuit in continuous renal replacement
therapy. Crit Care 2007;11:218. Crossref
11. Baldwin I. Factors affecting circuit patency and filter ‘life’.
Contrib Nephrol 2007;156:178-84. Crossref
12. Hetzel GR, Schmitz M, Wissing H, et al. Regional citrate
versus systemic heparin for anticoagulation in critically
ill patients on continuous venovenous haemofiltration: a
prospective randomized multicentre trial. Nephrol Dial
Transplant 2011;26:232-9. Crossref
13. Oudemans-van Straaten HM, Bosman RJ, Koopmans M,
et al. Citrate anticoagulation for continuous venovenous
hemofiltration. Crit Care Med 2009;37:545-52. Crossref
14. Fealy N, Baldwin I, Johnstone M, Egi M, Bellomo R. A
pilot randomized controlled crossover study comparing
regional heparinization to regional citrate anticoagulation
for continuous venovenous hemofiltration. Int J Artif
Organs 2007;30:301-7.
15. Kutsogiannis DJ, Gibney RT, Stollery D, Gao J. Regional
citrate versus systemic heparin anticoagulation for
continuous renal replacement in critically ill patients.
Kidney Int 2005;67:2361-7. Crossref
16. Monchi M, Berghmans D, Ledoux D, Canivet JL, Dubois
B, Damas P. Citrate vs. heparin for anticoagulation in
continuous venovenous hemofiltration: a prospective
randomized study. Intensive Care Med 2004;30:260-5. Crossref
17. Betjes MG, van Oosterom D, van Agteren M, van de
Wetering J. Regional citrate versus heparin anticoagulation
during venovenous hemofiltration in patients at low risk
for bleeding: similar hemofilter survival but significantly
less bleeding. J Nephrol 2007;20:602-8.
18. Zhang Z, Hongying N. Efficacy and safety of regional
citrate anticoagulation in critically ill patients undergoing
continuous renal replacement therapy. Intensive Care Med
2012;38:20-8. Crossref
19. Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW. Regional
citrate versus heparin anticoagulation for continuous
renal replacement therapy: a meta-analysis of randomized
controlled trials. Am J Kidney Dis 2012;59:810-8. Crossref
20. Liao YJ, Zhang L, Zeng XX, Fu P. Citrate versus
unfractionated heparin for anticoagulation in continuous
renal replacement therapy. Chin Med J (Engl)
2013;126:1344-9.
21. Khwaja A. KDIGO Clinical Practice Guidelines for Acute
Kidney Injury. Nephron Clin Pract 2012;120:179-84. Crossref
22. Zheng Y, Xu Z, Zhu Q, et al. Citrate pharmacokinetics in
critically ill patients with acute kidney injury. PLoS One 2013;8:e65992. Crossref
23. Meier-Kriesche HU, Gitomer J, Finkel K, DuBose
T. Increased total to ionized calcium ratio during
continuous venovenous hemodialysis with regional citrate
anticoagulation. Crit Care Med 2001;29:748-52. Crossref
24. Bakker AJ, Boerma EC, Keidel H, Kingma P, van der Voort
PH. Detection of citrate overdose in critically ill patients
on citrate-anticoagulated venovenous haemofiltration: use
of ionised and total/ionised calcium. Clin Chem Lab Med
2006;44:962-6. Crossref
25. Link A, Klingele M, Speer T, et al. Total-to-ionized calcium
ratio predicts mortality in continuous renal replacement
therapy with citrate anticoagulation in critically ill patients.
Crit Care 2012;16:R97. Crossref
26. Ward DM. The approach to anticoagulation in patients
treated with extracorporeal therapy in the intensive care
unit. Adv Ren Replace Ther 1997;4:160-73.
27. Saner FH, Treckmann JW, Geis A, et al. Efficacy and
safety of regional citrate anticoagulation in liver transplant
patients requiring post-operative renal replacement
therapy. Nephrol Dial Transplant 2012;27:1651-7. Crossref
28. Leung AK, Shum HP, Chan KC, Chan SC, Lai KY, Yan
WW. A retrospective review of the use of regional
citrate anticoagulation in continuous venovenous
hemofiltration for critically ill patients. Crit Care Res Pract
2013;2013:349512.
29. Shum HP, Chan KC, Yan WW. Regional citrate
anticoagulation in predilution continuous venovenous
hemofiltration using prismocitrate 10/2 solution. Ther
Apher Dial 2012;16:81-6. Crossref
30. Balik M, Zakharchenko M, Leden P, et al. Bioenergetic gain
of citrate anticoagulated continuous hemodiafiltration—a comparison between 2 citrate modalities and unfractionated heparin. J Crit Care 2013;28:87-95. Crossref
31. Leung AK, Yan WW. Renal replacement therapy in
critically ill patients. Hong Kong Med J 2009;15:122-9.
32. Takatori M, Yamaoka M, Nogami S, et al. Online CHDF
system: excellent cost-effectiveness for continuous
renal replacement therapy with high efficacy and
individualization. Contrib Nephrol 2010;166:173-80. Crossref
33. Szamosfalvi B, Frinak S, Yee J. Automated regional citrate
anticoagulation: technological barriers and possible
solutions. Blood Purif 2010;29:204-9. Crossref
34. Brandl M, Strobl K, Hartmann J, Kellner K, Posnicek
T, Falkenhagen D. A target-orientated algorithm for
regional citrate-calcium anticoagulation in extracorporeal
therapies. Blood Purif 2012;33:7-20. Crossref