Hong Kong Med J 2015 Apr;21(2):124–30 | Epub 10 Mar 2015
DOI: 10.12809/hkmj144292
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Comparison of efficacy and tolerance of short-duration open-ended ureteral catheter drainage and tamsulosin administration to indwelling double J stents following ureteroscopic removal of stones
Vikram S Chauhan, MB, BS, MS (Surgery)1;
Rajeev Bansal, MB, BS, MS (Surgery)1;
Mayuri Ahuja, MB, BS, DGO2
1 School of Medical Sciences & Research, Sharda University, Greater
Noida (U.P.) 201306, India
2 Kokila Dhirubhai Ambani Hospital & Medical Research Institute, Andheri West, Mumbai 400053, India
Corresponding author: Dr Vikram S Chauhan (vsing73@rediffmail.com)
Abstract
Objectives: To evaluate the efficacy of short-duration, open-ended ureteral catheter drainage as a replacement to indwelling stent, and to study the effect of tamsulosin on stent-induced pain and storage symptoms following uncomplicated ureteroscopic removal of stones.
Design: Prospective randomised study.
Setting: School of Medical Sciences and Research, Sharda University, Greater Noida, India.
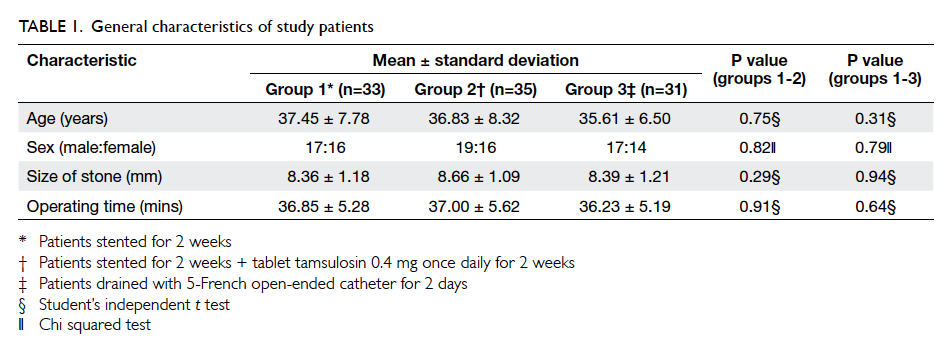
Patients: Patients who underwent ureteroscopic removal of stones for lower ureteral stones between November 2011 and January 2014 were randomly assigned into three groups. Patients in group 1 (n=33) were stented with 5-French double J stent for 2 weeks. Patients in group 2 (n=35) were administered tablet tamsulosin 0.4 mg once daily for 2 weeks in addition to stenting, and those in group 3 (n=31) underwent 5-French open-ended ureteral catheter drainage for 48 hours.
Main outcome measures: All patients were evaluated for flank pain using visual analogue scale scores at days 1, 2, 7, and 14, and for storage (irritative) bladder symptoms using International Prostate Symptom Score on days 7 and 14, and for quality-of-life score (using International Prostate Symptom Score) on day 14.
Results: Of the 99 patients, visual analogue scale scores were significantly lower for groups 2 and 3 (P<0.0001). The International Prostate Symptom Scores for all parameters were lower in patients from groups 2 and 3 compared with group 1 both on days 7 and 14 (P<0.0001). Analgesic requirements were similar in all three groups.
Conclusion: Open-ended ureteral catheter drainage is equally effective and better tolerated than routine stenting following uncomplicated ureteroscopic removal of stones. Tamsulosin reduces storage symptoms and improves quality of life after ureteral stenting.
New knowledge added by this
study
- This study shows that short-duration (up to 48 hours) ureteral drainage following ureteroscopic removal of stones (URS) has better efficacy and tolerance than indwelling stent placement with respect to the need for postoperative drainage. Hence, this can be a replacement for double J stenting.
- Routine tamsulosin administration in patients with indwelling stents following URS has beneficial effects not only on irritative bladder symptoms but also on flank pain (both persistent and voiding).
- Replacement of stents with short-duration open-ended ureteral catheter drainage provides early and more rehabilitation to the patients following URS. This is a viable option because there is no need for follow-up for stent-related symptoms, or maintaining records for planning its removal (no lost or retained stents).
- It avoids a second invasive endoscopic procedure of stent removal, thereby reducing the medical and financial burden on the patient (especially important in developing countries). Patients are more likely to undergo URS again if required in the future (with stone recurrence) than opt for less effective or expensive choices like medical management, shock wave lithotripsy, or alternative forms of medicine.
- In stented patients, tamsulosin administration improves the overall quality of life, and makes the period with stent in situ more bearable and asymptomatic.
Introduction
Ureteroscopic removal of stones (URS) is the
standard endoscopic method for treatment of lower
ureteric calculi. In recent times, this procedure does
not require routine dilatation of ureteric orifice due
to the availability of small-calibre rigid ureteroscopes
that can be easily manipulated into the ureter in
most of the cases.
Once the stones are removed, an indwelling
ureteral double J stent is placed which remains in
situ postoperatively for a period of 2 to 4 weeks.
This is dependent on a variety of factors such as the
difficulty in removal of stones, any mucosal injury,
and associated stricture of the ureter or its meatus.
Finney1 was the first to describe the use of double J
stents in the year 1978.2 The use of stents has proved
to be beneficial as seen in various studies, because
they prevent or reduce the occurrence of ureteric
oedema, clot colic, and subsequent development
of secondary ureteric stricture in cases with
mucosal injury or difficult stones.3 4 5 However, the
use of ureteral stents is not without its attendant
complications. Patients may develop flank pain,
haematuria, clot retention, dysuria, frequency, and
other irritative bladder symptoms following stent
placement in the postoperative period. Hence,
many authors have questioned the need for routine
placement of stents or their early removal.6 Recently,
researchers have proposed that the irritative and
other symptoms due to stents can be reduced or
overcome by the use of alpha blockers.7 With this
background knowledge, we conducted a prospective
randomised study with the aim to assess the efficacy
of oral tamsulosin for 14 days following stenting, and
efficacy of an open-ended ureteral catheter for 48
hours instead of a stent as viable options in patients
who underwent uncomplicated URS for lower
ureteric stones.
Methods
This study was conducted at School of Medical
Sciences and Research, Sharda University, Greater
Noida, India, after obtaining due clearance from the
ethics committee. Recruitment of patients was done
over a period from November 2011 to January 2014
and included a total of 99 patients who underwent
URS for lower ureteric stones.
Inclusion criteria were lower ureteric stones
defined as those imaged below the lower border
of sacroiliac joint of up to 10 mm in diameter on
computed tomography. Stones larger than 10 mm
in diameter, presence of ipsilateral kidney stones,
cases with lower ureteric or meatal stricture
requiring dilatation, and cases which had significant
mucosal injury (flap formation) per-operatively were
excluded.
All patients underwent URS under spinal
anaesthesia using an 8-French rigid
ureteroscope, and stones requiring fragmentation
were broken with a pneumatic lithoclast and these
fragments were retrieved with forceps. One surgeon
performed all the interventional procedures during
the study period.
The patients were randomly assigned to three
groups using randomisation table. On the random
number table, we chose an arbitrary place to start
and then read towards the right of the table from
that number. We used a number read on the table
from 1 to 3 to assign cases to group 1, a number from
4 to 6 to assign to group 2, and a number from 7 to 9
to assign cases to group 3 (a value of 0 was ignored).
A duty doctor prepared 120 serially numbered slips
of papers (indicating the number of enrolment) by
following the above randomisation protocol and had
written in them the group to which a new case was
to be assigned. The chits were folded, stapled, and
stacked in a box and stored in the operating theatre.
After completion of the URS, the floor nurse opened
the chit to reveal the appropriate enrolment number
and the group (group 1, 2 or 3) to which the patient
would go, thereby deciding further intervention.
Patients in group 1 underwent double J stent
placement following URS for a period of 2 weeks.
Patients in group 2 were administered tablet
tamsulosin 0.4 mg once daily for 2 weeks in addition
to double J stent. Patients in group 3 underwent
placement of an open-ended 5-French ureteral catheter
following the URS procedure, the distal end of which
was introduced into the lumen of Foley catheter.
Both the ureteric and Foley catheter were removed
on the second postoperative day in group 3 patients.
A 5-French 25-cm double J stent was used for
stenting and the duration of surgery was recorded
as time from the introduction of ureteroscope to
the placement of Foley catheter. Postoperatively,
patients were assessed for flank pain (persistent
or voiding) by asking them to report the pain on
a visual analogue scale (VAS) of 0 to 10 (0 being
no pain and 10 pain as severe as it could be) on
postoperative days 1, 2, 7, and 14. Patients were
also asked to report storage symptoms using the
International Prostate Symptom Score (IPSS) at
1 and 2 weeks postoperatively to assess irritative
bladder symptoms, while the IPSS quality-of-life
index was assessed at 2 weeks postoperatively.
All stented patients were discharged with tablet
levofloxacin 250 mg orally once daily for 2 weeks as
suppressive prophylaxis for infection.
Patients who had an indwelling double
J stent underwent stent removal after 2 weeks
by cystoscopy under local anaesthesia using 2%
lidocaine jelly supplemented with intravenous
injection of pentazocine 30 mg on a patient-need
basis, and were asked to report the pain experienced
during the stent removal on a VAS. Administration
and reporting of VAS scores was done by the floor
manager (administrative personnel) with assistance
from nurse on duty for the in-patients (wards),
while an intern and nurse on duty for out-patients
on follow-up was done in local language (Hindi). All
of these staff assessing VAS were blinded and had
no direct influence or active role in the treatment or
assessment protocol.
All patients on completion of 2 weeks of surgery
were asked, “Whether you would opt for the same
procedure again as treatment if you develop ureteral
stones in the future?” Patients complaining of pain
postoperatively were given injection tramadol 50 mg
intravenously if needed. If pain persisted, patients
were given intravenous injection of pentazocine 30
mg. All patients underwent intravenous urography
after 1 month of procedure to document stone
clearance and development of ureteral stricture.
Patients were asked to report to the out-patient
department if any other complications occurred
following discharge.
The sample size was estimated with the
following logic. We assumed the margin of error
that could be accepted as 5%, with a confidence
level of 90% and population size of 45 (cases that
were admitted with flank pain and require URS for
stones), in our institution the number of cases who
undergo URS typically in a year would be roughly
around 45 to 50. Assuming the response distribution
to be 50%, with the above assumptions, the sample
size calculated was 39, using the following formula:
Sample size n and margin of error E are given by
x = Z(c/100)2r(100 - r)
n = N x/((N - 1)E2 + x)
E = Sqrt[(N - n)x/n(N - 1)]
where N is the population size, r is the fraction of responses that we are interested in, and Z(c/100) is the critical value for the confidence level c.
Sample size n and margin of error E are given by
x = Z(c/100)2r(100 - r)
n = N x/((N - 1)E2 + x)
E = Sqrt[(N - n)x/n(N - 1)]
where N is the population size, r is the fraction of responses that we are interested in, and Z(c/100) is the critical value for the confidence level c.
This calculation is based on the normal
distribution, and assumes that there are more than
30 samples and a power of 80. Hence, we chose to
recruit approximately 35 patients in each arm of
study.
Statistical analyses
After collation of data, Student’s t test and Pearson
Chi squared test were used to analyse the three
groups for age, sex, stone size, and operating time.
We also comparatively evaluated the severity of
flank pain on postoperative days 1, 2 and weeks 1
and 2, and the IPSS for each group regarding storage
symptoms, total IPSSs at postoperative weeks 1 and
2, and the quality-of-life index at 2 weeks. Results
from groups 2 and 3 were compared with group 1 to
draw conclusions. Fisher’s exact test and Pearson
Chi squared tests were used to compare the number
of patients who needed intravenous analgesics due
to severe postoperative pain and to examine the
response to our question, “Whether you would opt
for the same procedure again as treatment if you
develop ureteral stones in the future?”
Results
There was no significant variation in the three
groups with regard to variables like age, sex, stone
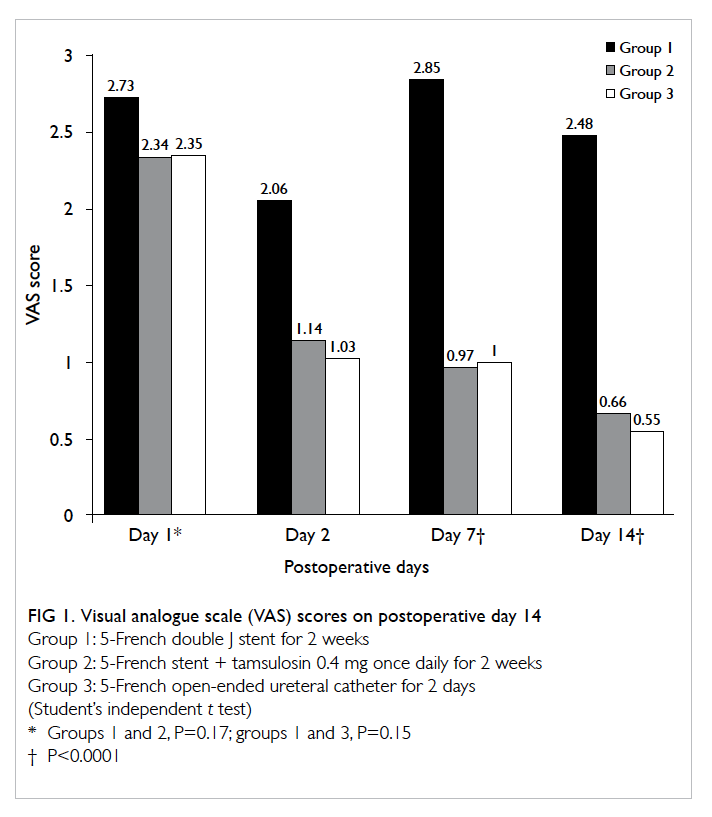
size, and operating time (Table 1). The VAS score for
flank pain, however, showed significant differences
among the three groups. On postoperative day 1, the
mean (± standard deviation) VAS scores in groups 1,
2, and 3 were 2.73 ± 1.14, 2.34 ± 1.12, and 2.35 ± 0.86
respectively, but were not statistically significant
(groups 1 and 2, P=0.17; groups 1 and 3, P=0.15).
On day 7, the mean VAS scores for groups 2 and 3
were 0.97 ± 0.77 and 1.00 ± 0.72 respectively, which
were significantly lower than group 1 score of 2.85 ±
1.52 (P<0.0001). On day 14, the mean VAS scores for
groups 1, 2, and 3 were 2.48 ± 1.40, 0.66 ± 0.67, and
0.55 ± 0.56 respectively (P<0.0001). This amounted
to significantly greater pain in group 1 patients as
compared with those in groups 2 and 3 (for groups 1-2
and 1-3, P<0.0001; Fig 1). Among those stented, the
mean VAS score for stent removal using 2% lidocaine
jelly was 3.76 ± 1.55 but the mean VAS score for stent
removal with regard to sex (male:female = 36:32) was
4.97 ± 0.80 and 2.41 ± 0.96, respectively and this was
statistically significant (P<0.0001).
Analyses of IPSS on both postoperative days
7 and 14 for bladder sensation, frequency, urgency,
nocturia, and the sum total of IPSS showed there
was significant decrease in group 2 as compared with
group 1 for all four parameters (P<0.0001). Group
3 patients had minimal mean IPSS scores to begin
with (Table 2). The mean quality-of-life scores for
groups 1, 2, 3 were 4.00 ± 0.92, 1.37 ± 0.86, and 0.52
± 0.50 respectively, and this was significantly better
for groups 2 and 3 compared with group 1 (P<0.00001;
Fig 2 and Table 3).
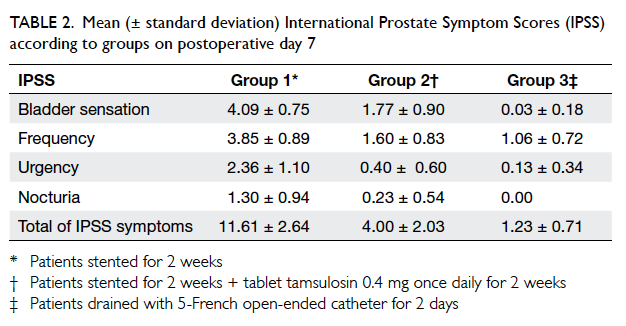
Table 2. Mean (± standard deviation) International Prostate Symptom Scores (IPSS) according to groups on postoperative day 7
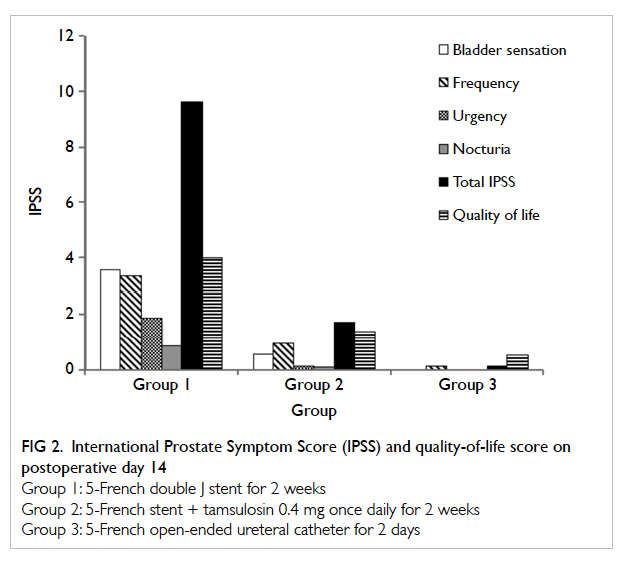
Figure 2. International Prostate Symptom Score (IPSS) and quality-of-life score on postoperative day 14
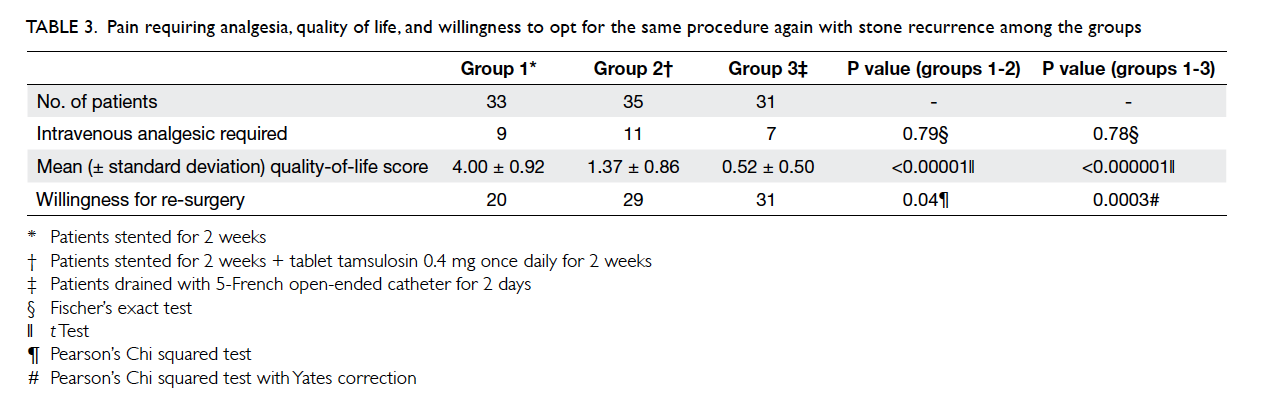
Table 3. Pain requiring analgesia, quality of life, and willingness to opt for the same procedure again with stone recurrence among the groups
Nine patients in group 1, 11 in group 2,
and seven in group 3 complained of pain requiring
injection of tramadol 50 mg (Table 3). Only
one patient (stent-only group) further required
intravenous injection of pentazocine 30 mg due to
persistent pain. No patient in any group required
intravenous analgesic after day 2 making analgesic
need similar in all groups. One patient who was
stented and had not received tamsulosin reported
gross haematuria on the sixth day, which required
readmission and catheterization with bladder wash,
and the haematuria responded to conservative
treatment. Beyond the 2-week period, no patient
reported any other complication during the 2-month
follow-up.
In this study, 20, 29, and all patients in groups
1, 2, and 3 respectively showed willingness for
undergoing same procedure in future if needed.
This showed that a higher percentage of patients
in groups 2 and 3 were willing for repeated surgery (if
needed) than in group 1, which was statistically
significant (for groups 1-2, P=0.04, and for groups
1-3, P=0.0003; Table 3). Two patients from the open
drainage group were lost to follow-up after 7 days.
There was no crossover from one group to the other
once assigned.
Discussion
Indwelling double J stents are routinely placed
following URS to prevent flank pain and secondary
ureteral strictures.4 8 9 However, duration-dependent
symptoms due to ureteral stents have been well
documented. Pollard and Macfarlane10 reported
stent-related symptoms in 18 (90%) out of 20 patients
who had indwelling ureteral stents following URS.
Bregg and Riehle11 reported that symptoms such
as gross haematuria (42%), dysuria (26%), and flank
pain (30%) appeared in stented patients prior to
being taken up for shock wave lithotripsy. Stoller et
al8 documented ureteral stent–related symptoms, like
flank pain, frequency, urgency, and dysuria, in at least
50% of patients who had an indwelling ureteral stent.
In a series by Han et al,12 haematuria was reported
as the most common symptom (69%) followed by
dysuria (45.8%), frequency (42.2%), lower abdominal
pain during voiding (32.2%), and flank pain (25.4%).
Most studies report that apart from urgency and
dysuria (which improve with time), there is no relief
in other symptoms till the stent is removed.
Wang et al7 showed that administration of α-blocker (tamsulosin) in stented patients improves flank pain and IPSS storage symptoms, along with an
overall improvement in quality of life. They reported
mean scores of frequency, urgency, nocturia as
3.7, 3.82, 2.01 in stented patients and 1.55, 1.43,
0.65 in those who received tamsulosin for 2 weeks,
respectively. The mean score of quality of life in IPSS
was 4.21 in stented group and 1.6 in stented + tamsulosin group. Moon et al13 reported that when compared
with stenting, all the storage categories of the IPSS
were significantly lower in the 1-day ureteral stent
group (P<0.01). Although the VAS scores were not
significantly different on postoperative day 1, it was
significantly lower in the 1-day ureteral catheter
group on postoperative days 7 and 14 (P<0.01).13
In our study, the mean total IPSS score at 2
weeks postoperatively was 9.64, 1.71, and 0.13 for
groups 1, 2, and 3 respectively (Fig 2). We also found
that the mean VAS scores for flank pain and the
mean IPSS scores of bladder sensation, frequency,
urgency, nocturia, were significantly higher in
patients in group 1 when compared with groups 2
and 3 (Figs 1 and 2). These findings suggest that the
indwelling double J stent causes time-dependent
pain and storage symptoms due to persistent
bladder irritation and administration of tamsulosin
did significantly decrease symptoms. Our patients
who received tamsulosin also fared much better
on the quality-of-life index at both 1 and 2 weeks
postoperatively than the group with stent placement
only (mean score, 1.37 and 4.00 respectively), while
those who underwent open-ended catheter drainage
showed minimal irritative symptoms (Table 2).
In addition, removal of indwelling stent
constitutes an additional procedure, which not only
is physical but also a financial burden to the patient
especially in a developing country like India. Kim
et al14 evaluated pain that occurred on cystoscopy
following an intramuscular injection of diclofenac
90 mg. The mean score of VAS during the procedure was
7.8 ± 0.7, which indicated severe pain. In
addition, only 22.5% of patients responded “yes” to
a questionnaire about their willingness to submit
to the same procedure again.14 Moon et al13 reported
a mean VAS score of 4.96 ± 1.29 for stent removal using
lidocaine gel. Although the mean VAS score for
stent removal under local anaesthesia in our series
was 3.76, the mean for males and females was 4.97
and 2.41, respectively. This amounts to moderately
severe pain in males, and in association with
irritative bladder symptoms that could influence the
patient’s willingness to go for a repeated procedure
in future if required. Besides, manipulation during
the procedure to remove the stent under local
anaesthesia especially in males could lead to urethral
or bladder injuries, a drawback that Hollenbeck et
al15 have observed.
Many have questioned the need for ureteral
stenting following URS. Denstedt et al16 in a series of
58 patients who underwent URS (29 stented and 29
non-stented) reported that there was no significant
difference in complications or success rates for URS
between stented versus non-stented cases. However,
Djaladat et al17 reported that when ureteroscopy
was performed without catheterization, flank pain
and renal colic could result from early ureteral
oedema implying that some postoperative drainage
is better than no drainage at all. This formed the
premise of using the open-ended ureteral catheter
in immediate postoperative period in our series and
the significantly lower VAS scores suggest that their
placement can be as effective as stents with minimal
irritative symptoms.17 Nabi et al18 concluded that
there was no significant difference in postoperative
requirements for analgesia, urinary tract infection,
the stone-free rate, or ureteric stricture formation
in patients who underwent uncomplicated URS.
There was no significant difference in analgesic
requirement in the three groups in our study; 9,
11, and 7 patients in groups 1, 2, and 3 respectively
required intravenous tramadol on postoperative days
1 and 2, only one patient in group 1 needed further
analgesia. No patient needed analgesics beyond the
second postoperative day which is comparable to
the series by Moon et al13 who reported that ratio
of patients who needed intravenous analgesics
because of severe postoperative flank pain was not
significantly different between stented and open-drainage
groups.
In our study, 20 out of 33 in group 1, 29 out
of 35 in group 2, and all 31 patients in group 3
responded affirmatively when asked “Whether you
would opt for the same procedure again as treatment
if you develop ureteral stones in the future?” The
P values for willingness for repeated procedure
were 0.04 and 0.0003 when comparing groups 1-2
and 1-3 respectively, which is in line with another
study (willingness P=0.02 in favour of open-ended
drainage).13 The results show that patients in groups
2 and 3 (tamsulosin and open-catheter drainage)
were significantly more likely to accept a repeated
procedure if needed. Hence, it can be inferred that
administration of tamsulosin following stenting or
placement of open-ended catheter (removed on day
2) was better tolerated by patients compared with an
indwelling stent–only procedure.
The relatively small sample size and being unblinded which was a likely placebo
effect in the tamsulosin group were the most obvious
limitations in our study. We believe that since in the stented
group patients were given tablet levofloxacin 250 mg
as suppressive prophylaxis post-discharge, any relief
in lower urinary tract symptoms therefore could
not be attributed to tamsulosin alone as placebo
effect. Assessment of VAS was done by personnel
who were blinded and had no direct influence on
the treatment or assessment protocol; this ruled out
surgeons’ bias and their involvement in influencing
the patient’s reporting of VAS scores. Degree of
difficulty, complexity, and duration of the procedure
could be construed as confounding factors in the
study. However, the relatively simple inclusion and
exclusion criteria which included all but the absolute
indications for stenting for comparison obviate this
and the results demonstrate that open-ended short-duration
ureteral drainage can replace stenting in all
other scenarios.
Conclusion
Accepting the limitations of a smaller sample size,
open-ended catheter drainage for 2 days is better
tolerated for flank pain and irritative bladder
symptoms when compared with an indwelling double J
stent for 2 weeks, without any significant difference
in complications or efficacy. We recommend this
procedure as a viable replacement to routine
stenting following URS. In those patients who do
undergo stenting following URS, administration
of tamsulosin significantly reduces stent-related
flank pain and irritative symptoms and enhances
the overall quality of life. In view of the possible
placebo effect on patients in group 2, the results
show that there is a need for more exhaustive and
larger multicentre randomised controlled trials to
assess the role of tamsulosin in countering post-URS stenting symptoms, given its wide acceptance
for pain relief and stone passage in treating lower
ureteral stones.
Declaration
No conflicts of interest were declared by authors.
References
1. Finney RP. Experience with new double J ureteral catheter
stent. J Urol 1978;120:678-81.
2. Hepperlen TW, Mardis HK, Kammandel H. Self-retained
internal ureteral stents: a new approach. J Urol
1978;119:731-4.
3. Lee JH, Woo SH, Kim ET, Kim DK, Park J. Comparison
of patient satisfaction with treatment outcomes between
ureteroscopy and shock wave lithotripsy for proximal
ureteral stones. Korean J Urol 2010;51:788-93. Crossref
4. Harmon WJ, Sershon PD, Blute ML, Patterson DE,
Segura JW. Ureteroscopy: current practice and long-term
complications. J Urol 1997;157:28-32. Crossref
5. Boddy SA, Nimmon CC, Jones S, et al. Acute ureteric
dilatation for ureteroscopy. An experimental study. Br J
Urol 1988;61:27-31. Crossref
6. Hosking DH, McColm SE, Smith WE. Is stenting following
ureteroscopy for removal of distal ureteral calculi
necessary? J Urol 1999;161:48-50. Crossref
7. Wang CJ, Huang SW, Chang CH. Effects of tamsulosin
on lower urinary tract symptoms due to double-J stent: a
prospective study. Urol Int 2009;83:66-9. Crossref
8. Stoller ML, Wolf JS Jr, Hofmann R, Marc B. Ureteroscopy
without routine balloon dilation: an outcome assessment. J
Urol 1992;147:1238-42.
9. Netto Júnior NR, Claro Jde A, Esteves SC, Andrade EF.
Ureteroscopic stone removal in the distal ureter. Why
change? J Urol 1997;157:2081-3. Crossref
10. Pollard SG, Macfarlane R. Symptoms arising from Double-J
ureteral stents. J Urol 1988;139:37-8.
11. Bregg K, Riehle RA Jr. Morbidity associated with
indwelling internal stents after shock wave lithotripsy. J
Urol 1989;141:510-2.
12. Han CH, Ha US, Park DJ, Kim SH, Lee YS, Kang SH.
Change of symptom characteristics with time in patients
with indwelling double-J ureteral stents. Korean J Urol
2005;46:1137-40.
13. Moon KT, Cho HJ, Cho JM, et al. Comparison of an
indwelling period following ureteroscopic removal
of stones between Double-J stents and open-ended
catheters: a prospective, pilot, randomized, multicenter
study. Korean J Urol 2011;52:698-702. Crossref
14. Kim KS, Kim JS, Park SW. Study on the effects and safety
of propofol anaesthesia during cytoscopy. Korean J Urol
2006;47:1230-5. Crossref
15. Hollenbeck BK, Schuster TG, Faerber GJ, Wolf JS Jr.
Routine placement of ureteral stents is unnecessary after
ureteroscopy for urinary calculi. Urology 2001;57:639-43. Crossref
16. Denstedt JD, Wollin TA, Sofer M, Nott L, Weir M, D’A
Honey RJ. A prospective randomized controlled trial
comparing nonstented versus stented ureteroscopic
lithotripsy. J Urol 2001;165:1419-22. Crossref
17. Djaladat H, Tajik P, Payandemehr P, Alehashemi S. Ureteral
catheterization in uncomplicated ureterolithotripsy: a
randomized, controlled trial. Eur Urol 2007;52:836-41. Crossref
18. Nabi G, Cook J, N’Dow J, McClinton S. Outcomes of
stenting after uncomplicated ureteroscopy: systematic
review and meta-analysis. BMJ 2007;334:572. Crossref