Hong Kong Med J 2015 Feb;21(1):38–44 | Epub 21 Nov 2014
DOI: 10.12809/hkmj144241
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Role of fine-needle aspiration cytology in human immunodeficiency virus–associated lymphadenopathy: a cross-sectional study from northern India
Naveen Kumar, MD1; BB Gupta, MD1; Brijesh Sharma, MD1; Manju Kaushal, MD2; BB Rewari, MD1; Deepak Sundriyal, MD1
1 Department of Medicine, PGIMER and Dr RML Hospital, New Delhi 110001, India
2 Department of Pathology, PGIMER and Dr RML Hospital, New Delhi 110001, India
Corresponding author: Dr Naveen Kumar (docnaveen2605@yahoo.co.in), (2605docnaveen@gmail.com)
Abstract
Objective: To evaluate the role of fine-needle
aspiration cytology in the diagnosis of human
immunodeficiency virus (HIV)–associated
lymphadenopathy.
Design: Case series.
Setting: Tertiary care teaching hospital, India.
Patients: Fifty consecutive HIV-positive patients,
who presented with lymphadenopathy at the out-patient
department and antiretroviral therapy clinic.
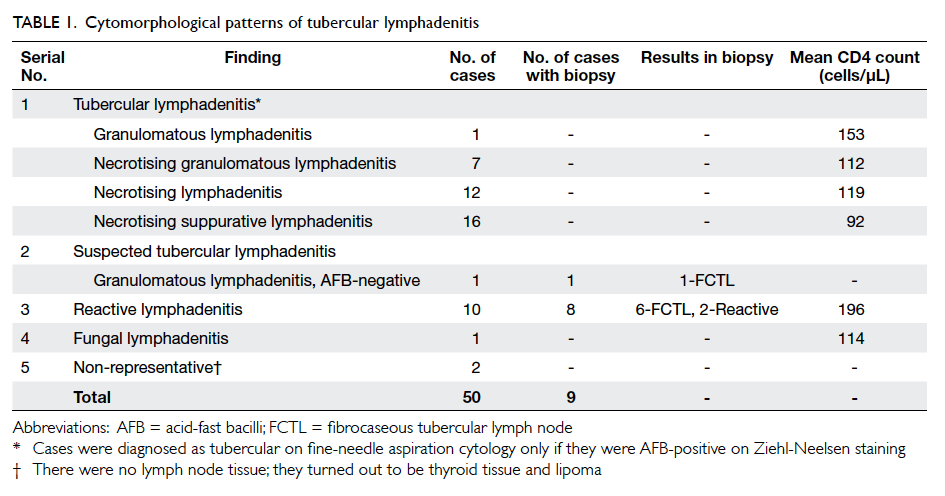
Results: Tubercular lymphadenitis was the most
common diagnosis, reported in 74% (n=37) of
patients; 97.2% of them were acid-fast bacilli–positive. Reactive lymphadenitis and fungal
lymphadenitis were present in 10 and 1 cases,
respectively. The most common cytomorphological
pattern of tubercular lymphadenitis was necrotising suppurative
lymphadenitis, present in 43.2% (n=16)
of patients. Of eight biopsies done in reactive cases,
six turned out to be tubercular lymphadenitis. Fine-needle
aspiration cytology had a sensitivity of 83.7%
for diagnosing tubercular lymphadenitis.
Conclusion: Necrotising suppurative lymphadenitis
should be recognised as an established pattern of
tubercular lymphadenitis. Reactive patterns should
be considered inconclusive rather than a negative
result, and re-evaluated with lymph node biopsy.
Fine-needle aspiration cytology is an excellent test
for diagnosing tubercular lymphadenitis in HIV-associated
lymphadenopathy.
New knowledge added by this
study
- Necrotising suppurative lymphadenitis should be recognised as an established pattern of tubercular lymphadenitis.
- In advanced human immunodeficiency virus (HIV) disease, reactive lymphadenitis should be considered inconclusive rather than a negative result, and re-evaluated with lymph node biopsy.
- As lymphadenopathy is common in all stages of HIV disease, judicious use of fine-needle aspiration cytology can be helpful in diagnosing associated opportunistic infections and other pathological conditions.
Introduction
Human immunodeficiency virus (HIV) infection
is an important worldwide public health problem.
Developing nations, where resources are limited, are
the worst affected nations. Until curative treatment
for HIV infection becomes available, the crux of
management is early diagnosis and treatment with
highly active antiretroviral therapy.
As HIV is a lymphotropic virus, lymphoid
tissues are the major anatomical site where the
virus establishes itself during early infection. These
lymphoid tissues act as reservoirs for the virus in
the asymptomatic phase of infection. In the late
stage, HIV disseminates from these sites to cause a
full-blown acquired immunodeficiency syndrome
(AIDS).1 Thus, lymph node involvement is found
in all stages of infection. The cause of lymph node
enlargement is often difficult to establish by history,
physical examination, radiographic studies, and
routine laboratory tests. Surgical biopsy is the gold
standard for diagnosis. However, it has several
drawbacks: costly, time-consuming, and requiring
more elaborate precautions. Fine-needle aspiration
cytology (FNAC) does not have any of these
limitations, and is also comparatively less invasive.
Furthermore, the cost of aspiration cytology is only
10% to 30% of that of surgical biopsy.2
We performed FNAC to establish the
aetiological diagnosis in our study subjects with HIV
infection. To detect false-negative results, biopsy was
done in cases diagnosed as reactive lymphadenitis.
The aims of the study were to assess the accuracy of
FNAC and to correlate the findings with clinical and
laboratory parameters like CD4 counts.
Methods
The study was conducted in the Departments of
Medicine and Pathology, PGIMER and Dr RML
Hospital, New Delhi, India, from January 2009 to
December 2009. The study protocol and proforma
were approved by the ethics committee of the
institute. Informed written consent was obtained
from all patients. Fine-needle aspiration cytology
was performed in 50 consecutive HIV-positive
patients presenting with lymphadenopathy at the
antiretroviral clinic, or the out-patient or in-patient
services. Detailed history was taken and examination
of the patients was performed. Clinical stage (as per
the World Health Organization [WHO] classification)
and CD4 counts were recorded for all patients. Fine-needle
aspiration cytology was performed by the
clinician on the largest non-inguinal lymph node
using standard precautions. The area was cleaned
and draped. A 10-mL syringe and 23-gauge needles
were used. If the sample was insufficient, another
sample was taken from a different lymph node. Slides
for Papanicolaou and Periodic-acid Schiff (PAS)
stains were fixed with 95% ethanol immediately after
preparing the smear; others were air dried. A total of
six slides were prepared from each aspirate and were
immediately processed by staining with Giemsa
stain, Papanicolaou’s stain, Ziehl-Neelsen (ZN) stain
for acid-fast bacilli (AFB), PAS stain for fungi, and
Gram stain. Cases that were AFB-positive on ZN
staining were diagnosed as tubercular lymphadenitis;
otherwise, they were retained as suspected cases.
Based on the presence or absence of granulomas,
caseation (necrosis) and neutrophilic infiltration,
tubercular lymph nodes were classified into four
cytomorphological categories: granulomatous
lymphadenitis (GL), necrotising granulomatous
lymphadenitis (NGL), necrotising lymphadenitis
(NL), and necrotising suppurative lymphadenitis
(NSL). Lymph node biopsies were performed in
cases which showed a reactive pattern or suspected
tubercular lymphadenitis on FNAC. Sensitivity,
specificity, and positive and negative predictive
values were calculated for FNAC as a diagnostic
modality compared with biopsy. Statistical analysis
for association between FNAC findings and
various parameters was done using univariate
and multivariate logistic regression analyses. Data
analysis was performed by the Statistical Package for the
Social Sciences (Windows version 19.0; SPSS Inc,
Chicago [IL], US). A P value of less than 0.05 was
regarded as statistically significant.
Results
A total of 50 patients (43 men and 7 women) were
included in the study. The mean age of the patients
was 32.4 years. Cervical region was the most
common site of lymphadenopathy (n=39; 78%)
followed by axillary and inguinal regions. The lymph
nodes were matted and generalised in 62% (n=31)
and 48% (n=24) of cases, respectively. Generalised
lymphadenopathy was present in 54% (n=20) of
cases with tubercular lymphadenitis and 40% (n=4)
of cases with reactive lymphadenitis. Nature of
aspirate was bloody in 21 (42%) cases, caseous in 24
(48%), and mixed (with blood and caseation) in the
remaining patients. The CD4 count ranged from
12 cells/µL to 353 cells/µL, with a mean count of 131
cells/µL. Most of the patients were in WHO clinical
stage 3 (n=31; 62%).
The most common cytological diagnosis was
tubercular lymphadenitis (n=37; 74%) followed by
reactive pattern (n=10; 20%). Only one FNAC was
diagnosed as fungal lymphadenitis showing PAS-positive
spores of Histoplasma capsulatum (Fig 1). In two cases, the cytologies were suggestive of thyroid tissue and lipoma; these were treated as failed FNACs. Tubercular lymphadenitis was further categorised into four cytomorphological patterns, as shown in Table 1. All tubercular cases were AFB-positive except one which was a AFB-negative GL on FNAC. Subsequently, this was shown to be AFB-positive tubercular lymphadenitis on biopsy. Of the 10 cases reported as reactive lymph nodes on FNAC, eight gave consent for biopsy. Biopsy showed AFB-positive fibrocaseous tubercular lymphadenopathy in six out of these eight cases; in the remaining
two cases, biopsy findings matched with the FNAC findings.
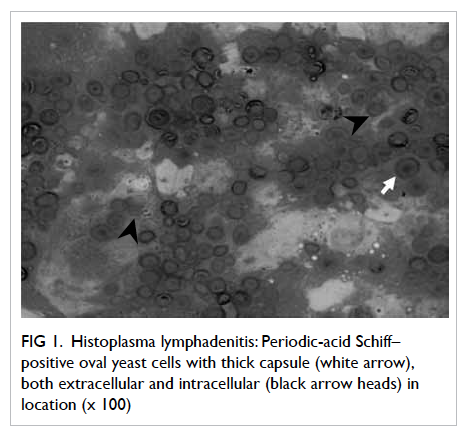
Figure 1. Histoplasma lymphadenitis: Periodic-acid Schiff–positive oval yeast cells with thick capsule (white arrow), both extracellular and intracellular (black arrow heads) in location (x 100)
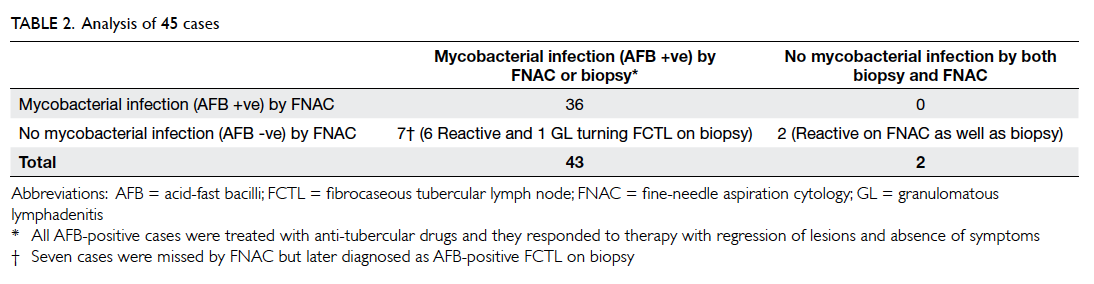
All cases diagnosed as having mycobacterial
disease on FNAC and those who underwent biopsy
were included in the analysis. Hence 45 cases were
analysed: 36 cases diagnosed as mycobacterial
(tubercular) lymphadenitis on FNAC, one case of
GL which was AFB-positive fibrocaseous tubercular
lymph node on biopsy, and eight cases of reactive
lymphadenopathy that underwent biopsy (Table 2). The sensitivity and negative predictive value of
FNAC for diagnosing tubercular lymphadenitis were
83.7% and 22.2%, respectively. As AFB positivity was the requisite criterion for diagnosing
tubercular lymphadenitis, it was expected that there
would be no diagnosis of tuberculosis (TB) in any
case which was AFB-negative on FNAC; thus, the
specificity and positive predictive value were 100%.
We performed logistic regression analysis with
tubercular lymphadenitis as the dependent variable
and four parameters as covariates. On univariate
analysis, CD4 count (P=0.016), nature of aspirate
(P=0.013), and matted nodes on examination
(P=0.028) were associated with tubercular aetiology
on FNAC; lymph node distribution did not show any
such association (P=0.401). However, in multivariate
analysis, none of these factor was associated with
tubercular aetiology on FNAC. Moreover, none of
these factors was associated with the severe form
(NL or NSL) of tubercular lymphadenitis either on
univariate or multivariate analysis.
Discussion
Lymphadenopathy in HIV patients is very
common; it can be a presenting feature in about
35% of patients with AIDS.3 Causes can be varied,
depending on the stage of the disease, and may
include persistent generalised lymphadenopathy,
lymphoid malignancies, and opportunistic infection.
All these can be easily and efficiently diagnosed
by aspiration study of these lymph nodes. These
causes of lymphadenopathies are important causes
of death in AIDS patients. In this study, we aimed
to investigate the performance of FNAC for the
accurate diagnosis of lymphadenopathies. We also
compared our results with those from similar Indian
and western studies (Table 3
1 4 5 6 7 8 9 10 11 12 13 14 15 16).
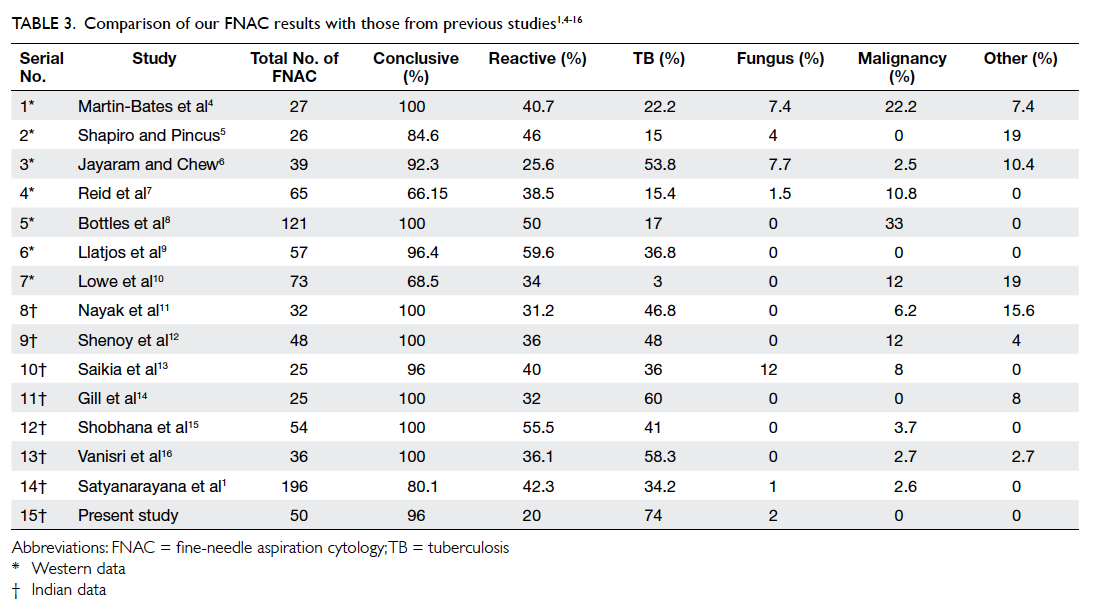
Table 3. Comparison of our FNAC results with those from previous studies1 4 5 6 7 8 9 10 11 12 13 14 15 16
Tuberculosis is the most frequent opportunistic infection in HIV patients.17 18 Lymph nodes are the commonest site of extra-pulmonary TB in patients with AIDS.19 20 Using FNAC as the diagnostic modality, we also found tubercular lymphadenitis to be the most common cause of lymphadenopathy, present in 74% of our patients. Similar conclusion was drawn in other Indian studies; however they reported a prevalence of 34.2% to 60% (Table 3). The high prevalence of tubercular lymphadenitis in our series may be related to the low immunity of the majority of patients; 41 out of 50 patients had CD4 counts of <200 cells/µL.
There are two specific pathological criteria for
diagnosing tubercular lymphadenitis—caseation
and granuloma formation. Both are less likely to
be present in tubercular lymphadenitis associated
with advanced HIV disease. This is because T-cell
function, which is suppressed in advanced HIV
disease, is required for granuloma formation. On the
basis of these two findings, tubercular lymphadenitis
is classified into three categories21 22: GL, NGL, and
NL.
The GL pattern can occur due to several
causes. However, in a country like India, where TB
is very common, this pattern is considered to be
due to TB until proven otherwise. We found this
pattern in 5.4% (2 out of 37 cases with tubercular
lymphadenitis) of TB cases, a finding similar to that
in other previous studies where it ranged from 4.3%
to 28.5% (Table 4).6 9 11 12 16 23 The NGL pattern, with
both caseation and epithelioid granulomas, is the
most typical pattern of tubercular lymphadenitis. It
was present in 18.9% (7 out 37 cases of tubercular
lymphadenitis) of our cases; other studies have
reported it in the range of 26.6% to 74% (Table 4).9 11 12 16 23 Necrotising lymphadenitis represents
the most severe cytomorphological pattern of tubercular lymphadenitis. There is complete
necrosis with only ‘acellular’ debris. It is not labelled as ‘purulent’ because there are no degenerated
polymorphonuclear cells. Complete necrosis reflects impaired cell-mediated immunity in this group of
patients. Cases of NGL can be wrongly labelled as NL if material is aspirated from that part of the node
which contains only caseation. This was the second most common pattern reported in our study (32.4%;
12 out of 37 cases of tubercular lymphadenitis). In other studies, the reported prevalence rates range
from 8.6% to 57.1% (Table 4).6 9 11 12 16 23
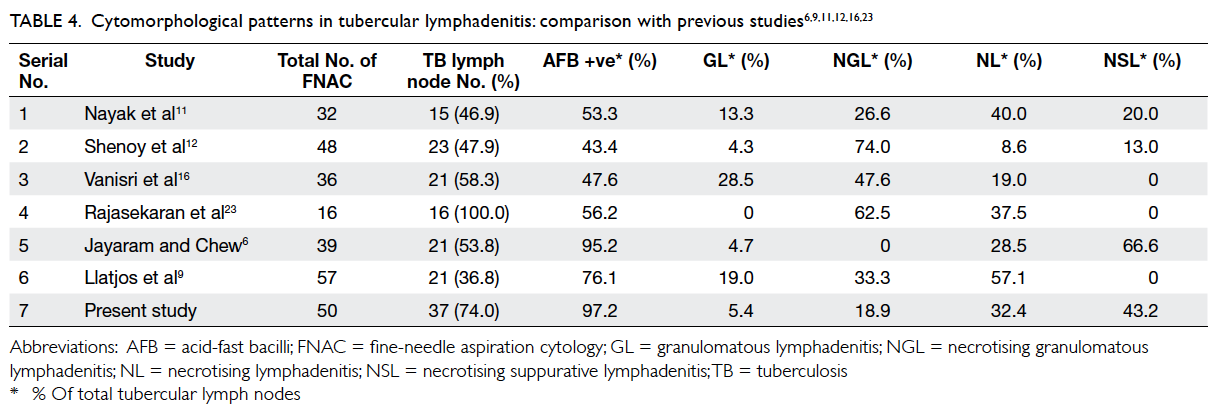
Table 4. Cytomorphological patterns in tubercular lymphadenitis: comparison with previous studies6 9 11 12 16 23
The NSL pattern of tubercular lymphadenitis
(Fig 2) was the most common cytomorphological
picture, seen in 43.2% (16 out of 37 cases of tubercular lymphadenitis) of patients. This pattern
was reported in 20% and 13% cases of tubercular lymphadenitis by Nayak et al11 and Shenoy et al,12 respectively (Table 4). Jayaram and Chew6 reported this pattern in 67% of their TB cases in Kuala Lumpur, Malaysia. Although reported in these case series, unlike the other three patterns, the NSL pattern is not yet a well-recognised cytomorphological type of tubercular lymphadenitis. However, this pattern is important, especially in HIV patients. If ZN staining is not done, the thin caseation commonly present in these cases can be mistaken for pus, and the case wrongly labelled as pyogenic lymphadenitis. Similar observations have been made in some studies.6 11 12 24
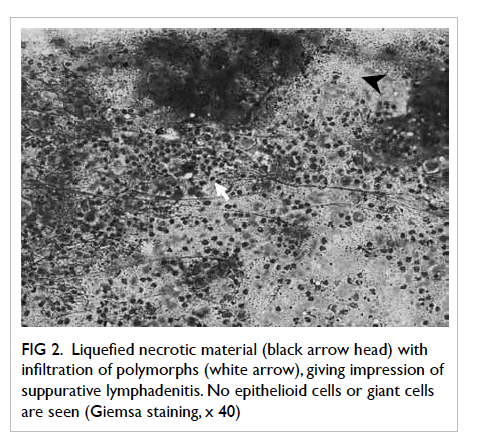
Figure 2. Liquefied necrotic material (black arrow head) with infiltration of polymorphs (white arrow), giving impression of suppurative lymphadenitis. No epithelioid cells or giant cells are seen (Giemsa staining, x 40)
Studies have shown that FNAC is more sensitive for the diagnosis of TB in HIV-positive patients than in seronegative patients.25 In our series, AFB positivity rate in TB cases was 97.2% (n=36/37),
which was higher than that in previous studies (43.4% to 95.2%).6 9 11 12 16 This could be due to the fact that the disease was quite advanced in our group of tubercular lymphadenitis patients (mean CD4 count of 108 cells/µL). Moreover, as the cytomorphological pattern deteriorated and necrosis appeared, AFB positivity increased from 50% to 100%. This was in agreement with data from earlier studies.6 9 11 12 16 Chances of detecting AFB were least in lymph nodes
showing GL pattern: four out of five studies6 9 11 12 16 did not report any AFB-positive case with this pattern of lymphadenopathy. Further, although TB is very common in HIV subjects, Mycobacterium avium complex (MAC) is not frequently seen in India. Its chance further decreases by adding MAC prophylaxis of azithromycin to the patient’s treatment regimen.
A reactive lymphadenitis was observed in only 20% of cases in our study. Most of the western studies reported it as the most common lymph node pathology, observed in 25.6% to 59.6% of cases (Table 3). One case of histoplasmosis was detected in our
study (Fig 1). On Giemsa staining, the lymph
node showed reactive lymphoid cells, histiocytes, areas of granuloma formation, along with sheets
of Histoplasma capsulatum organism, located both extracellularly and intracellularly, which were
positive on PAS staining. Hence, although the finding of GL without AFB in HIV-infected patients
in India is taken as TB unless proven otherwise, causes like fungal infection (by PAS staining) should
be excluded, especially if the CD4 count is low.
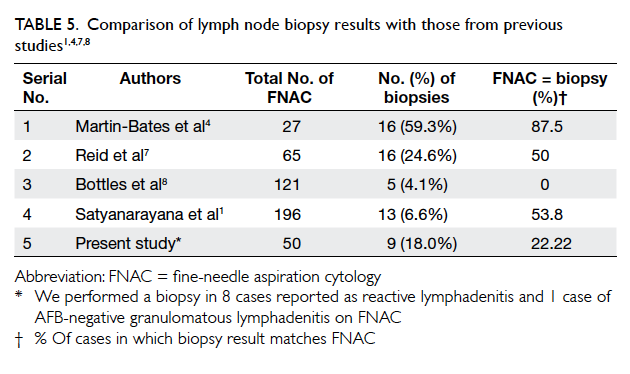
We performed a lymph node biopsy in 18% of our cases (Table 5). Among these, the findings
were different from those in FNAC in 77.8% of the cases. False-negative rate in our study was 16.3% (7
out of 43 cases of TB; Table 2). The false-negative rate in other studies ranges from 2% to 9.2%.1 4 7 8 Of these seven false-negative cases, six were diagnosed
as reactive nodes on FNAC which later showed fibrocaseous nodes on biopsy. Possible reasons for
discordance could be focal tubercular involvement of the nodes. On FNAC, the tubercular area could
have been missed and, hence, wrongly labelled as reactive cases. Also, different nodes in the same area can enlarge due to different pathologies. Hence, a report of reactive pattern in advanced disease, like in our group of patients (mean CD4 in reactive group being 196 cells/µL), does not have much value, and should not be the end of further assessment. It should be considered an inconclusive result rather
than a negative one. It emphasises the importance of performing a biopsy in this group of patients.
A falling CD4 count in our group of patients
was associated with increasing risk of tubercular
lymphadenitis. However, CD4 counts did not
predict the severity of cytomorphological forms
of tubercular lymphadenitis. Of note, 41 out of 50
patients had CD4 counts of <200 cells/µL. Hence,
we did not have a group of patients with higher
CD4 counts in whom less severe forms of tubercular
lymphadenitis were more common. This association
should be studied further by recruiting patients with
a wide range of CD4 counts.
In univariate analysis, a matted lymph node
on examination (P=0.028) and caseous material
(P=0.013) on aspiration were found more often in TB
cases versus reactive cases. Hence, apart from routine
cytology stains, these observations guide us for
ordering special staining like ZN staining. However,
their association with cytomorphological pattern
of TB was not significant on univariate analysis, as
the whole spectrum of pattern can have caseation
on aspiration (except GL) and matted nodes on
examination, although cytomorphologically these
are of increasing severity.
Conclusion
Tuberculosis is the most common aetiology of
HIV-associated lymphadenopathy in India. Acid-fast
bacilli positivity is very high in HIV-associated
tubercular lymphadenitis. We recommend routine
AFB staining for all lymph nodes undergoing FNAC
in HIV patients. Lymph nodes showing AFB-negative
GL pattern on FNAC should be stained for
fungus, especially if CD4 count is low. If a patient’s
CD4 count is low, a reactive FNAC pattern should be
taken as an inconclusive result and is an indication
for biopsy. All lymph nodes showing NSL pattern on
FNAC should undergo ZN staining in HIV-positive
patients. It should be recognised as a tubercular
cytomorphological pattern, especially in patients
with low immunity like those with AIDS. Fine-needle
aspiration cytology of lymph nodes is a valuable test
for diagnosing tubercular lymphadenitis in HIV-associated
lymphadenopathy.
References
1. Satyanarayana S, Kalighatgi AT, Murlidhar A, Prasad RS,
Jawed KZ, Trehan A. Fine needle aspiration cytology of
lymph node in HIV infected patients. Medical Journal
Armed Forces India 2002;58:33-7. CrossRef
2. Kaminsky DB. Aspiration biopsy for community hospital.
In: Johnston WW, editor. Masson monograph in
diagnostic cytopathology. New York: Masson Publication; 1981: 12-3.
3. Guidelines for prevention and management of common
opportunistic infection/malignancy among HIV-infected
adult and adolescents. NACO. Ministry of Health & Family
Welfare. Government of India; May 2007.
4. Martin-Bates E, Tanner A, Suvarna SK, Glazer G, Coleman
DV. Use of fine needle aspiration cytology for investigating
lymphadenopathy in HIV positive patients. J Clin Pathol
1993;46:564-6. CrossRef
5. Shapiro AL, Pincus RL. Fine-needle aspiration of diffuse
cervical lymphadenopathy in patients with acquired
immunodeficiency syndrome. Otolaryngol Head Neck
Surg 1991;105:419-21.
6. Jayaram G, Chew MT. Fine needle aspiration cytology
of lymph nodes in HIV-infected individuals. Acta Cytol
2000;44:960-6. CrossRef
7. Reid AJ, Miller RF, Kocjan GI. Diagnostic utility of fine
needle aspiration (FNA) cytology in HIV-infected patients
with lymphadenopathy. Cytopathology 1998;9:230-9. CrossRef
8. Bottles K, McPhaul LW, Volberding P. Fine-needle aspiration
biopsy of patients with acquired immunodeficiency
syndrome (AIDS): experience in an outpatient clinic. Ann
Intern Med 1988;108:42-5. CrossRef
9. Llatjos M, Romeu J, Clotet B, et al. A distinctive cytologic
pattern for diagnosing tuberculous lymphadenitis in AIDS.
J Acquir Immune Defic Syndr 1993;6:1335-8.
10. Lowe SM, Kocjan GI, Edwards SG, Miller RF. Diagnostic
yield of fine-needle aspiration cytology in HIV-infected
patients with lymphadenopathy in the era of highly active
antiretroviral therapy. Int J STD AIDS 2008;19:553-6. CrossRef
11. Nayak S, Mani R, Kavatkar AN, Puranik SC, Holla VV.
Fine-needle aspiration cytology in lymphadenopathy of
HIV-positive patients. Diagn Cytopathol 2003;29:146-8. CrossRef
12. Shenoy R, Kapadi SN, Pai KP, et al. Fine needle aspiration
diagnosis in HIV related lymphadenopathy in Mangalore,
India. Acta Cytol 2002;46:35-9. CrossRef
13. Saikia UN, Dey P, Jindal B, Saikia B. Fine needle aspiration
cytology in lymphadenopathy of HIV-positive cases. Acta
Cytol 2001;45:589-92. CrossRef
14. Gill PS, Arora DR, Arora B, et al. Lymphadenopathy.
An important guiding tool for detecting hidden HIV-positive cases: a 6-year study. J Int Assoc Physicians AIDS
Care (Chic) 2007;6:269-72. CrossRef
15. Shobhana A, Guha SK, Mitra K, Dasgupta A, Neogi DK,
Hazra SC. People living with HIV infection / AIDS—a study on lymph node FNAC and CD4 count. Indian J Med Microbiol 2002;20:99-101.
16. Vanisri HR, Nandini NM, Sunila R. Fine-needle aspiration
cytology findings in human immunodeficiency virus
lymphadenopathy. Indian J Pathol Microbiol 2008;51:481-4. CrossRef
17. Harries AD. Tuberculosis and human immunodeficiency
virus infection in developing countries. Lancet
1990;335:387-90. CrossRef
18. Sharma SK, Kadhiravan T, Banga A, Goyal T, Bhatia I,
Saha PK. Spectrum of clinical disease in a series of 135
hospitalised HIV-infected patients from north India. BMC
Infect Dis 2004;4:52. CrossRef
19. Shafer RW, Kim DS, Weiss JP, Quale JM. Extrapulmonary
tuberculosis in patients with human immunodeficiency
virus infection. Medicine (Baltimore) 1991;70:384-97. CrossRef
20. Arora VK, Kumar SV. Pattern of opportunistic pulmonary
infections in HIV sero-positive subjects: observations
from Pondicherry, India. Indian J Chest Dis Allied Sci
1999;41:135-44.
21. Das DK, Pant JN, Chachra KL, et al. Tuberculous
lymphadenitis: correlation of cellular components and
necrosis in lymph-node aspirate with A.F.B. positivity and
bacillary count. Indian J Pathol Microbiol 1990;33:1-10.
22. Das DK. Fine needle aspiration cytology in diagnosis of
tuberculous lesion. Lab Med 2000;31:625-32. CrossRef
23. Rajasekaran S, Gunasekaran M, Jayakumar DD, et al.
Tuberculous cervical lymphadenitis in HIV positive and
negative patients. Indian J Tuberc 2001;48:201-4.
24. Havlir DV, Barnes PF. Tuberculosis in patients with
human immunodeficiency virus infection. N Engl J Med
1999;340:367-73. CrossRef
25. Shriner KA, Mathisen GE, Goetz MB. Comparison of
mycobacterial lymphadenitis among persons infected with
human immunodeficiency virus and seronegative controls.
Clin Infect Dis 1992;15:601-5. CrossRef
Find HKMJ in MEDLINE: