DOI: 10.12809/hkmj134095
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Post-transplantation primary central nervous system lymphoma in a patient with systemic lupus erythematosus and prolonged use of immunosuppressant
Teresa PK Tse, MB, ChB; Allan NL Chan, FHKCEM, FHKAM (Emergency Medicine); Tony KT Chan, FCSHK, FHKAM (Surgery); YC Po, FCSHK, FHKAM (Surgery)
Department of Neurosurgery, Princess Margaret Hospital, Lai Chi Kok,
Hong Kong
Corresponding author: Dr Teresa PK Tse (teresapoki@hotmail.com), (tpk730@ha.org.hk)
Abstract
Post-transplantation primary central nervous system
lymphoma is an uncommon and fatal post-transplant
lymphoproliferative disorder. Such lymphomas
have been described in only a few case series in the
literature. The incidence of this condition is rising
with improved survival after organ transplantation. A
case of post-transplantation primary central nervous
system lymphoma in a young Chinese woman
with systemic lupus erythematosus is described
here. She presented with right-sided weakness and
memory loss after tooth extraction 2 weeks before
admission. Contrast computed tomography of the
brain demonstrated a contrast rim-enhancing lesion
over the left frontal lobe. With a history of recent
dental procedure, long-term immunosuppressive
therapy and computed tomography findings,
cerebral abscess was highly suspected. Emergency
operation was performed. Histopathology showed
post-transplantation primary central nervous system
lymphoma, with cells positive for B-cell marker
CD20. Immunosuppressant was stopped and she
was treated with radiotherapy and rituximab (anti-CD20 monoclonal antibody). She remained disease-free at 16 months. Post-transplantation primary
central nervous system lymphoma is rare with
variable presentation and radiological features. We
believe rituximab may have a role in the treatment of
such lymphomas.
Introduction
Post-transplant lymphoproliferative disorder
(PTLD) is a rare neoplastic complication of solid
organ transplantation, affecting less than 2% of
post-transplant patients. It includes a spectrum of
diseases ranging from Epstein-Barr virus (EBV)–driven polyclonal lymphoid proliferation to EBV-positive
or -negative malignant lymphoma. Post-transplantation
primary central nervous system
lymphoma (PT-PCNSL) is an uncommon and
potentially fatal PTLD that develops in post-transplantation
patients with the tumours confined
to the brain and spinal cord, affecting 10% of patients
with PTLD, which in turn affects only 1% of patients
with kidney transplantation. The most common
form of PCNSL is diffuse large B-cell lymphoma.1 2
To date, PT-PCNSL has been described in only case
reports and a few case series in the literature.3 4 The
exact incidence of PT-PCNSL is unknown, but it is
expected to be rising in the future with improving
survival for patients with organ transplant.5 Clinical
presentation and radiological features of PT-PCNSL
can vary. Here we describe a case of PT-PCNSL in a
Chinese woman with systemic lupus erythematosus
(SLE) and prolonged use of immunosuppressant.
Case report
A young woman with a known history of SLE
underwent cadaveric renal transplantation for end-stage
renal failure at the age of 28 years. She developed
a complication of moderate cellular rejection
postoperatively and was placed on mycophenolate
mofetil (MMF) 750 mg every morning and 500 mg
in the afternoon, and prednisolone 5 mg daily since
2000. Her renal function worsened after an episode
of acute pyelonephritis in 2010 with creatinine level
rising to 210 mg/dL from 150 mg/dL. She remained
well afterwards until December 2011 when she was
admitted to our hospital for progressive right-sided
weakness and memory loss after tooth extraction 2
weeks before admission. On physical examination,
she was found to have expressive dysphasia and
right-sided weakness. Urgent contrast computed
tomography (CT) of the brain demonstrated a 3.9
cm x 6.4 cm x 4.7 cm multilobulated contrast rim-enhancing
lesion in the left frontal region with
perifocal oedema and midline shift (Fig 1). With a history of recent dental procedure, long-term
immunosuppressive therapy, and CT findings,
cerebral abscess was highly suspected. Emergency
operation was arranged and intravenous antibiotics
were started immediately.
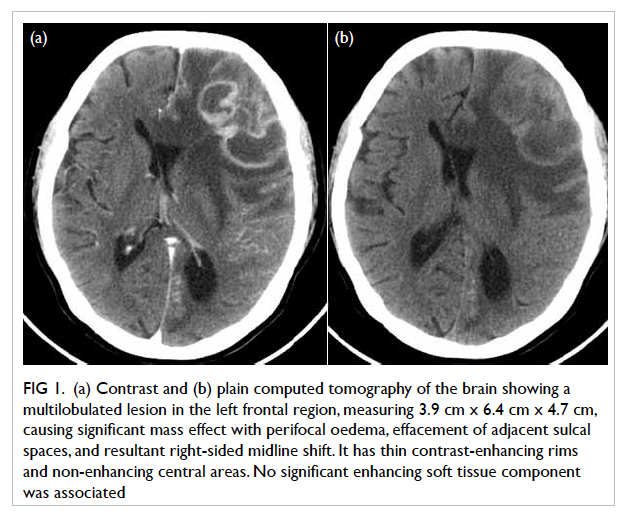
Figure 1. (a) Contrast and (b) plain computed tomography of the brain showing a multilobulated lesion in the left frontal region, measuring 3.9 cm x 6.4 cm x 4.7 cm, causing significant mass effect with perifocal oedema, effacement of adjacent sulcal spaces, and resultant right-sided midline shift. It has thin contrast-enhancing rims and non-enhancing central areas. No significant enhancing soft tissue component was associated
Operation
Burr hole for tapping of abscess was planned initially.
Intra-operative ultrasound revealed an isodense
lesion underneath the dura. Tapping was performed
thrice but no fluid was aspirated. As frozen section
was unavailable during non-office hours, we
decided to perform left frontal craniotomy. Frontal
lobectomy with partial excision of lesion was done.
The lesion was found to be rubbery, lobulated, and
non-vascular.
Pathological findings
Pathological examination revealed a lympho-proliferative
lesion characterised by extensive
infiltration by abnormal medium–to–large-sized
lymphoid cells with large areas of necrosis. The
abnormal lymphoid cells were monomorphic with
vesicular nuclei and small nucleoli. The neoplastic
cells were strongly positive for B-cell marker CD20.
They were also positive for BCL2 and CD30, but
negative for CD10 and T-cell marker CD3. In
addition, the tumour cells were positive for EBV-encoded
early RNAs (EBER) and EBV LMP-1. The
Ki-67 proliferation index was estimated at 40% to
50%. The morphological findings, supported by
immunohistochemical studies, were consistent with
monomorphic PTLD, primary diffuse large B-cell
lymphoma of the central nervous system (CNS) [Fig
2].
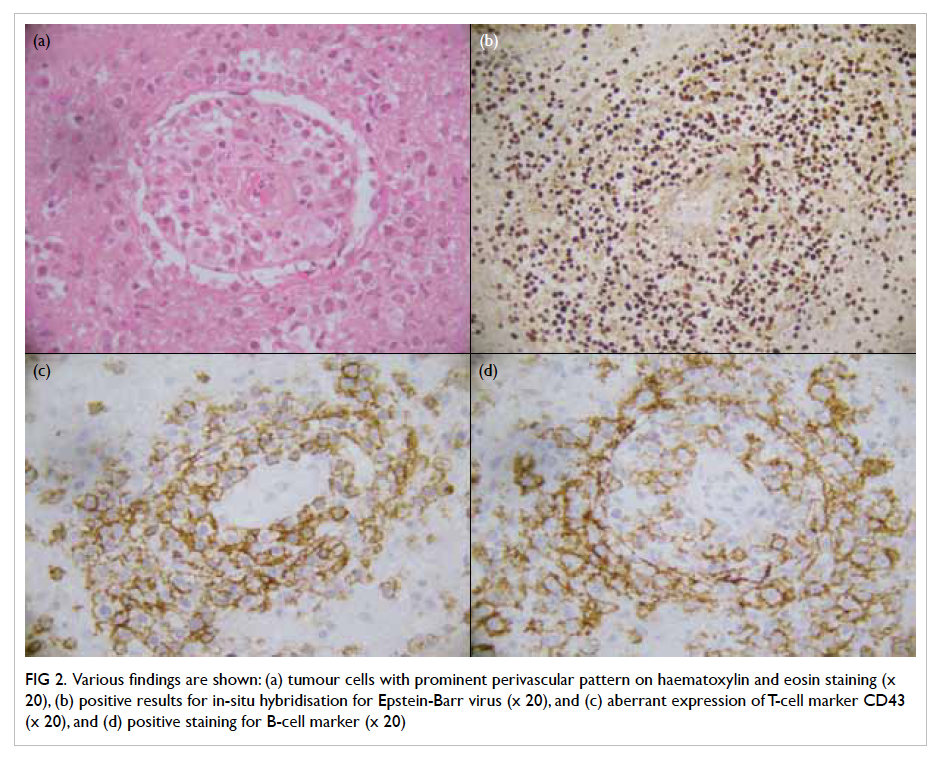
Figure 2. Various findings are shown: (a) tumour cells with prominent perivascular pattern on haematoxylin and eosin staining (x 20), (b) positive results for in-situ hybridisation for Epstein-Barr virus (x 20), and (c) aberrant expression of T-cell marker CD43 (x 20), and (d) positive staining for B-cell marker (x 20)
Postoperative course
Further workup showed that the patient had
isolated CNS lymphoma. Bilateral bone marrow
biopsies were done which showed no evidence of
lymphoproliferative disease. Postoperative positron
emission tomography–computed tomography
(PET-CT) revealed residual hypermetabolic left
frontal lymphomatous deposits but there were no
hypermetabolic foci in the neck, thorax, abdomen,
and pelvis. Serology was negative for EBV all along.
Postoperatively, the patient was continued on
prednisolone and her antibiotics were discontinued.
Mycophenolate mofetil was stopped and she was
started on everolimus 0.25 mg daily. In view of
suboptimal Karnofsky Performance Score and
deteriorating renal function, she was treated with
whole-brain radiotherapy (WBRT) alone (40 Gy/20
fr) followed by rituximab consolidation therapy (500
mg, once every 3 week, for 4 weeks). Five months
after surgery, PET-CT showed complete resolution
of the left frontal hypermetabolic foci; PET-CT 16
months after surgery showed stable disease. She is
currently doing well 30 months after operation.
Discussion
Post-transplantation PCNSL
is a rare neoplasm. Its clinical presentation and
radiological features can vary. In a case series
that involved 33 patients with PT-PCNSL imaged
by contrast magnetic resonance imaging (MRI),
41% had homogeneously enhanced lesions, while
29% had ring enhancement and 61% had multiple
lesions.6 In a review involving 221 patients with
ring-enhancing lesions on MRI, 40% were gliomas,
30% were brain metastases, 12% were brain
abscesses, 6% were multiple sclerosis plaques, and
2% were lymphomas.7 Imaging modalities such as
magnetic resonance spectroscopy (MRS) may aid in
differentiating PCNSL from brain abscess. In MRS,
PCNSL typically demonstrates a lipid peak with
raised choline to N-acetylacetate (NAA) ratio; while
abscess typically demonstrates a lactate peak with
reduced choline and NAA. Both PCNSL and abscess
demonstrate restricted diffusion in diffusion-weighted
imaging. Nuclear imaging such as PET
scan may also help by showing high uptake in PCNSL
while the uptake is low in abscess. However, urgent
MRI, MRS, and PET scan were not readily available
in our centre during non-office hours. In our case,
the patient presented with focal neurological deficit
with a history of recent dental procedure, use of
long-term immunosuppressive therapy, and contrast
rim-enhancing lesion on CT. The overall picture was
suggestive of cerebral abscess, which warranted
urgent surgical drainage.
Systemic lupus erythematosus is associated
with an increased risk of haematological cancer,
mainly non-Hodgkin’s lymphoma, while association
with PCNSL is very rare with only few case reports
on the condition in the literature. Moreover,
most of these cases were associated with serious
immunosuppressive therapy. Possible risk factors of
PT-PCNSL include high-dose immunosuppressant
and negative EBV serology in the transplant recipient.8
Our patient developed PT-PCNSL after kidney
transplantation with prolonged use of MMF, and her
EBV serology was also negative. It is postulated that
EBV seronegativity and immunosuppression may
predispose the transplant recipient to a novel EBV
infection and, thus, the development of PT-PCNSL.
However, the association of PT-PCNSL and SLE
remains unclear.
The best treatment of PT-PCNSL has not
been established. Reduction of immunosuppressive
therapy, WBRT, and chemotherapy with agents
like methotrexate and rituximab have been used
for treating patients with PT-PCNSL. Whole-brain
radiotherapy induced complete response by
neuroimaging in 60% of patients with PCNSL but the
median overall survival was only 12 months.9 High-dose
intravenous methotrexate is now the standard
of care for PCNSL with reported overall survival
of up to 60 months.10 Rituximab, an anti-CD20
monoclonal antibody, has been used to treat patients
with systemic PTLD. As rituximab does not penetrate
the blood-brain barrier effectively, its effectiveness
in treating PT-PCNSL is doubtful.11 Only three
studies involving 10 patients with PT-PCNSL treated
with intravenous rituximab have been reported
with overall survival of at least 20 months.6 12 13
Resection of PCNSL has been discouraged as it
causes significant neurological deficit without any
survival benefit. In our case, after partial resection
of tumour, WBRT and rituximab were used to treat
PT-PCNSL. The patient remained disease-free at 16
months with MRI showing complete resolution of
the lesions; she remains asymptomatic at 30 months
after operation. It is believed that rituximab may
have a role in the management of patients with PT-PCNSL
by achieving adequate drug penetration into
the brain parenchyma through leaky lymphomatous
vasculature. We propose reconsidering the
statement that efforts at resection of PCNSL should
be discouraged, at least if resection seems safe. Yet,
further studies are required to determine the best
treatment for PT-PCNSL.
Conclusion
Post-transplantation PCNSL is a rare neoplasm with
variable clinical presentation and radiological features.
Possible risk factors include EBV seronegativity and
prolonged use of immunosuppressive therapy. We
believe rituximab and tumour resection may have a
role in the treatment of PT-PCNSL.
Acknowledgement
We would like to express our special thanks to Dr
WL Lam for the pathological examination of the
specimen.
Declaration
No conflicts of interest were declared by authors.
References
1. Castellano-Sanchez AA, Li S, Qian J, Lagoo A, Weir E,
Brat DJ. Primary central nervous system posttransplant
lymphoproliferative disorders. Am J Clin Pathol
2004;121:246-53. CrossRef
2. Vaglio A, Manenti L, Mancini C, et al. EBV-associated
leukoencephalopathy with late onset of central nervous
system lymphoma in a kidney transplant recipient. Am J
Transplant 2010;10:947-51. CrossRef
3. Phan TG, O’Neill BP, Kurtin PJ. Posttransplant primary
CNS lymphoma. Neuro Oncol 2000;2:229-38. CrossRef
4. Snanoudj R, Durrbach A, Leblond V, et al. Primary brain
lymphomas after kidney transplantation: presentation and
outcome. Transplantation 2003;76:930-7. CrossRef
5. Wolfe RA, Roys EC, Merion RM. Trends in organ donation
and transplantation in the United States, 1999-2008. Am J
Transplant 2010;10:961-72. CrossRef
6. Cavaliere R, Petroni G, Lopes MB, Schiff D; International
Primary Central Nervous System Lymphoma
Collaborative Group. Primary central nervous system
post-transplantation lymphoproliferative disorder: an
International Primary Central Nervous System Lymphoma
Collaborative Group Report. Cancer 2010;116:863-70. CrossRef
7. Schwartz KM, Erickson BJ, Lucchinetti C. Pattern of
T2 hypointensity associated with ring-enhancing brain
lesions can help to differentiate pathology. Neuroradiology
2006;48:143-9. CrossRef
8. Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott
K. Posttransplant lymphoproliferative disorders after renal
transplantation in the United States in era of modern
immunosuppression . Transplantation 2005;80:1233-43. CrossRef
9. Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s
lymphoma of the brain: can high dose, large volume
radiation therapy improve survival? Report on a
prospective trial by the Radiation Therapy Oncology
Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys
1992;23:9-17. CrossRef
10. Thiel E, Korfel A, Martus P, et al. High-dose methotrexate
with or without whole brain radiotherapy for primary CNS
lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority
trial. Lancet Oncol 2010;11:1036-47. CrossRef
11. Ruhstaller TW, Amsler U, Cerny T. Rituximab: active
treatment of central nervous system involvement by non-Hodgkin’s lymphoma? Ann Oncol 2000;11:374-5. CrossRef
12. Traum AZ, Rodig NM, Pilichowska ME, Somers MJ.
Central nervous system lymphoproliferative disorder in
pediatric kidney transplant recipients. Pediatr Transplant
2006;10:505-12. CrossRef
13. Kordelas L, Trenschel R, Koldehoff M, Elmaagacli A,
Beelen DW. Successful treatment of EBV PTLD with CNS
lymphomas with the monoclonal anti-CD20 antibody
rituximab. Onkologie 2008;31:691-3. CrossRef

