Hong Kong Med J 2014 Dec;20(6):486–94 | Epub 7 Nov 2014
DOI: 10.12809/hkmj144246
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
The benefit of prothrombin complex concentrate in decreasing neurological deterioration in patients with warfarin-associated intracerebral haemorrhage
WC Fong, FRCP, FHKAM (Medicine)1;
WT Lo, MRCP, FHKAM (Medicine)1;
YW Ng, MRCP, FHKAM (Medicine)1;
YF Cheung, MPH, FHKAM (Medicine)1;
Gordon CK Wong, MRCP, FHKAM (Emergency Medicine)2;
HF Ho, FRCS, FHKAM (Emergency Medicine)2;
John HM Chan, FRCP, FHKAM (Medicine)1;
Patrick CK Li, FRCP, FHKAM (Medicine)1
1 Division of Neurology, Department of Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Department of Accident and Emergency, Queen Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr WC Fong (fwcz01@ha.org.hk)
Abstract
Objective: To compare the outcomes of patients
with warfarin-associated intracerebral haemorrhage
given different treatments to reverse the effect of
anticoagulation.
Design: Historical cohort study.
Setting: A regional hospital in Hong Kong.
Patients: Patients on warfarin who developed
intracerebral haemorrhage.
Interventions: Prothrombin complex concentrate
versus fresh frozen plasma treatment.
Main outcome measures: The primary outcome
measures included the international normalised ratio
before and after prothrombin complex concentrate
treatment and the neurological deterioration in
patients with Glasgow Coma Scale score of more than
8/not intubated/not planned for immediate surgery
(target group). Secondary outcome measures were
haematoma expansion, 7-day and 30-day mortality
rates, and 3-month functional outcome. Safety
outcome was the occurrence of a thrombotic event
after prothrombin complex concentrate treatment
within the index admission.
Results: Among 33 patients with clearly
documented time of infusion of prothrombin
complex concentrate, and whose international
normalised ratio was checked before and after
prothrombin complex concentrate treatment, the
mean international normalised ratio was reduced
from 2.81 to 1.21 within 24 hours. Within the target
group of patients, there was a significantly lower rate
of neurological deterioration in the prothrombin
complex concentrate group (17.4% of 23 patients)
versus fresh frozen plasma group (45.5% of 33
patients) [P=0.027]. In terms of the 7-day mortality,
30-day mortality, and 3-month functional outcome,
prothrombin complex concentrate–treated group
showed a favourable trend although the difference
did not reach a statistical significance. No patient
developed thrombotic complications after
prothrombin complex concentrate treatment.
Conclusions: Prothrombin complex concentrates
can reverse the warfarin effect of prolonged
international normalised ratio in a timely manner.
It might better improve the outcome of warfarin-associated
intracerebral haemorrhage compared
with fresh frozen plasma treatment by reduction in
neurological deterioration.
New knowledge added by this
study
- Outcome of warfarin-associated intracerebral haemorrhage might be improved by prothrombin complex concentrate treatment.
- Prothrombin complex concentrate should be considered the first-line reversal agent for patients with warfarin-associated intracerebral haemorrhage unless contra-indicated.
Introduction
Warfarin-associated intracerebral haemorrhage
(ICH) is associated with high mortality of 40% to 60%.
Compared with spontaneous ICH, it has a higher
mortality rate and poorer functional outcome.1 2 Up
to 50% of patients will have haematoma expansion
within 24 hours, and the haematoma expansion
time interval is more prolonged versus those with
spontaneous ICH.3 Predictors of poor outcome
include age, Glasgow Coma Scale score (GCS)
on admission, baseline haematoma volume, and
haematoma expansion2 4; of these, haematoma
expansion is the only predictor that can be modified
upon admission. Controlling/limiting haematoma
expansion, thus, serves as the target for improving
the outcome.
Prompt reversal of anticoagulation has been
found to decrease haematoma expansion, especially
if it can be reversed within 2 hours of admission.5 6
The available agents for this include vitamin K,
fresh frozen plasma (FFP), prothrombin complex
concentrates (PCC), and recombinant factor VII
(rFVIIa). Vitamin K should be given to all patients
for sustained international normalised ratio (INR)
reversal; however, given by itself, it is associated
with a slow onset of action. Prothrombin complex
concentrate contains factor II, IX, X (3-factor), and
some preparations also have factor VII (4-factor).
If only 3-factor PCC are available, as in the case of
hospitals in Hong Kong, it has been recommended
to give additional FFP to replenish factor VII levels.7 8
Prothrombin complex concentrates are preferred
over FFP alone for warfarin reversal as these can be
given quickly without any need for cross-matching
of blood or thawing, and have a much smaller
volume of infusion, decreasing the risk of volume
overload. The onset of action is much faster than
FFP and the INR can be reversed in as early as 15
minutes after infusion; in contrast, FFP may take
hours for INR reversal. Prothrombin complex
concentrates are readily available, are less expensive
than rFVIIa, and have a longer half-life than rFVIIa.1
Many international societies recommend PCC as the
first-line agents for warfarin reversal in emergency
situations.9 10 11 12 Those recommendations are, however,
mainly based on rapid reversal of INR by PCC.
Currently, there is no prospective, randomised study
comparing PCC versus FFP in terms of the long-term
functional outcomes. In Hong Kong, PCC are
not commonly used for warfarin reversal, probably
because these have not been proven to be superior
to FFP in large-scale studies, and are associated with
a low but definite risk of thrombosis.
Our hospital started formal implementation
of PCC protocol for warfarin-associated ICH in
2011, initially in the Department of Medicine,
and subsequently in the Accident and Emergency
Department and Department of Neurosurgery
(Fig 1) [while PCC were used in 2010, a formal
protocol was not implemented until 2011]. The PCC
preparation in our hospital was Prothrombinex-HT,
and was later supplied as Prothrombinex-VF (3-factor; CSL Behring, Broadmeadows, Australia). Our
protocol recommends the prompt use of the 3-factor
PCC with dosage based on INR level, together with
intravenous injection of 10 mg vitamin K and 2
units of FFP upon diagnosis, and rechecking INR
30 minutes after administration of PCC; additional
warfarin reversal agents are given provided the INR
remains high.
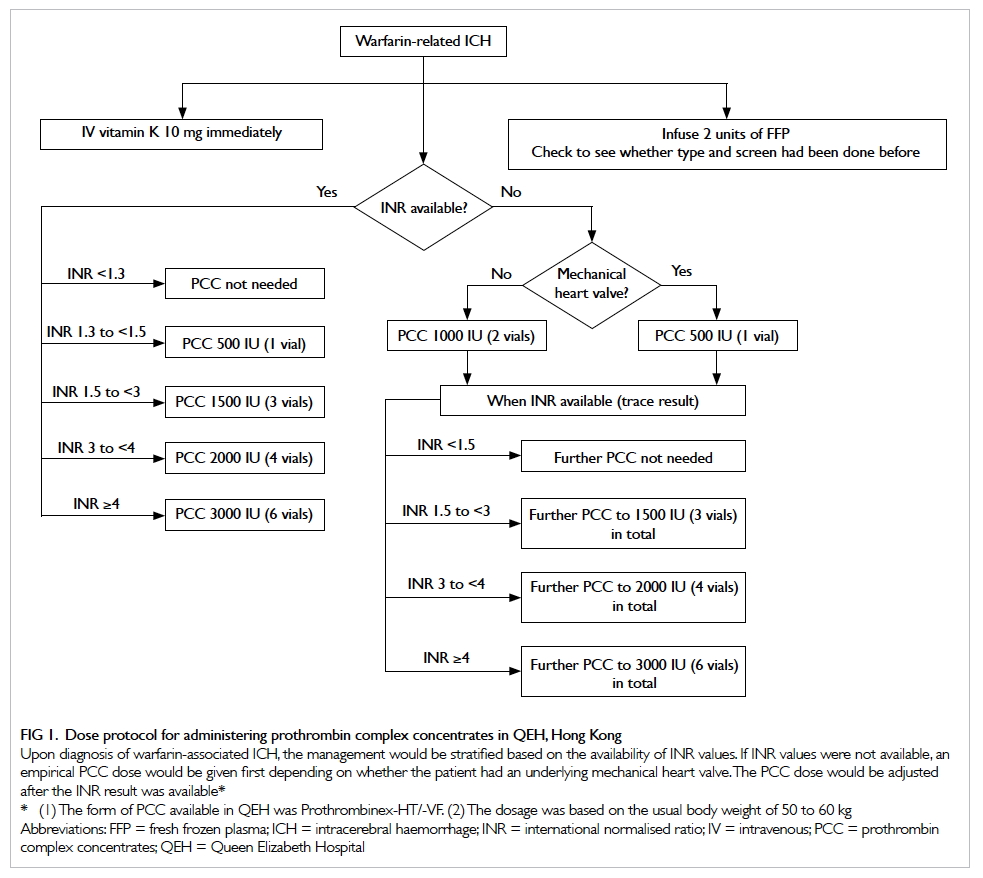
Figure 1. Dose protocol for administering prothrombin complex concentrates in QEH, Hong Kong
Upon diagnosis of warfarin-associated ICH, the management would be stratified based on the availability of INR values. If INR values were not available, an empirical PCC dose would be given first depending on whether the patient had an underlying mechanical heart valve. The PCC dose would be adjusted after the INR result was available
The objectives of this study were to review
the use of PCC in our hospital (including the use,
dosage, complications) and to compare the clinical
outcomes with patients given FFP treatment, with
the ultimate aim of looking for indirect evidence of
benefit of PCC over FFP treatment.
Methods
This was a retrospective review of patients admitted
to Queen Elizabeth Hospital, a tertiary hospital in
Hong Kong. Data on patients with ICH and use of
warfarin prior to admission were retrieved from the
Clinical Data Analysis and Reporting System. Data of
patients who were treated with PCC from February
2011 (start of implementation of PCC protocol)
to September 2013 were analysed. This was the
PCC group. Similarly, data from January 2007 to
September 2013 were reviewed and data of patients
given treatment other than PCC were analysed. Only
patients given FFP were included in the comparison
arm. This was the FFP group.
Patients given no reversal agents or solely
given vitamin K were not included in this study.
One patient given rFVIIa was excluded. Patients
with known or suspected secondary causes of
bleeding such as tumour bleeding, underlying subacute
bacterial endocarditis, and vasculitis were excluded.
Patients with haemorrhagic transformation of
infarct, subdural haemorrhage, and subarachnoid
haemorrhage were excluded. One patient who was
started on warfarin reversal agent in another hospital
and later transferred to our hospital after a few days
was also excluded.
From the case records, all data including
demographics (age, sex, indications for
anticoagulation, concomitant use of antiplatelet
agent, co-morbidities such as diabetes, hypertension,
ischaemic heart disease, prior stroke), clinical
state at the time of admission (GCS, limb power),
management including reversal agents used (vitamin
K, FFP, PCC), intubation, and whether surgery
had been performed were reviewed. Laboratory
data (INR on admission and after PCC use) and
radiological data (baseline haematoma volume and
follow-up computed tomography [CT] brain scans)
were reviewed from the Electronic Patient Record
system. The CT scans were reviewed by two authors
who were not blinded to the treatment information,
while the intracerebral haematoma volume was
calculated based on the ABC/2 score.13 Haematoma
volume within the ventricular extension was
estimated by using the intraventricular haemorrhage
score.14
The primary outcome measure was
neurological deterioration, defined as ≥2-point
decrease in GCS or limb power. This was selectively
studied in patients with an initial GCS of more
than 8, who were not intubated and who were not
initially considered for surgery upon diagnosis of
ICH, ie those who were initially selected for medical
treatment (target group). The rationale for this was
that the patients who have the maximum benefit
from rapid reversal agents are those with initial
good GCS, for whom the agents can prevent further
deterioration. Patients with poor GCS due to large
ICH upon presentation probably do not benefit much
from rapid reversal. The secondary clinical outcome
measures were haematoma expansion (defined as
>33% increase in haematoma volume),15 7-day and
30-day mortality rates, and modified Rankin scale
(mRS) on 3 months of follow-up.
Statistical analysis was performed using the
Statistical Package for the Social Sciences (Windows
version 20; SPSS Inc, Chicago [IL], US). Proportion
between groups was compared using the Fisher’s
exact test, and continuous variables were compared
using the Wilcoxon rank sum test. P value was
considered significant if it was less than 0.05.
Results
Figure 2 shows the recruitment of patients with
warfarin-associated ICH identified before and
after protocol implementation and the subsequent
formation of cohorts for analysis.
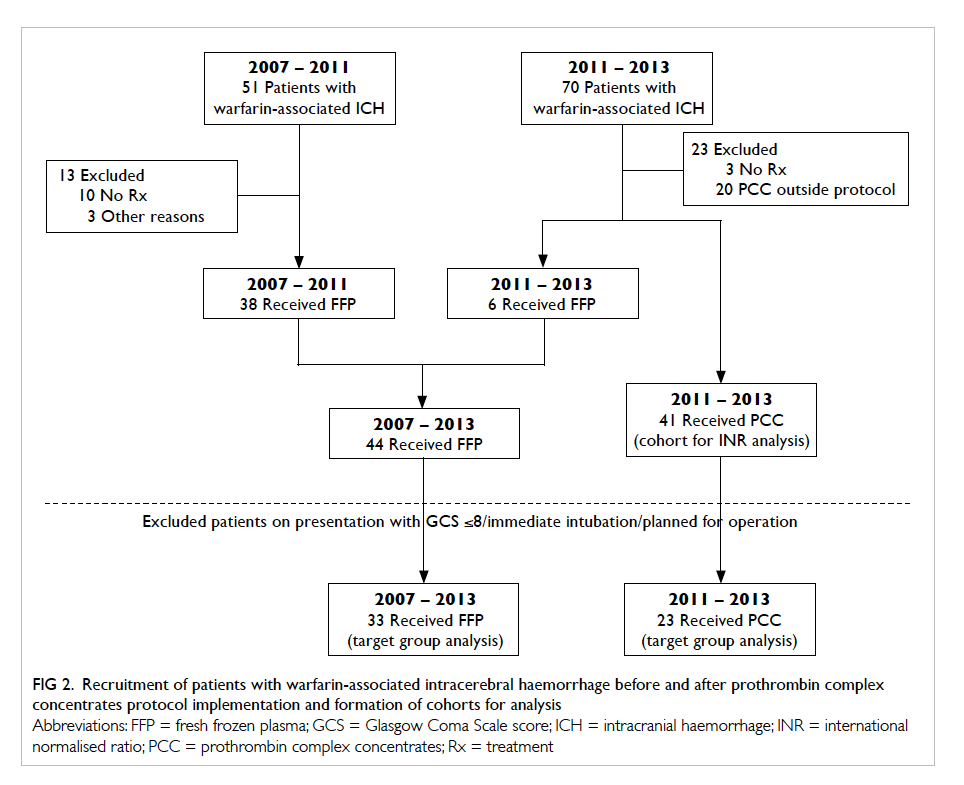
Figure 2. Recruitment of patients with warfarin-associated intracerebral haemorrhage before and after prothrombin complex concentrates protocol implementation and formation of cohorts for analysis
Analysis of use of prothrombin complex
concentrates (from February 2011 to
September 2013 after implementation of
protocol)
Of 70 patients who had warfarin-associated ICH,
61 were given PCC, and nine patients were not
given PCC for the following reasons: too ill for
undergoing CT brain when stroke was suspected,
and stroke confirmed only afterwards (n=1); nearly
moribund state on admission (n=6; one of these
had concomitant allergy to FFP and two had recent
pulmonary embolism/deep vein thrombosis); and
small ICH size (0.07 cm3) and symptom onset more
than 1 week ago with a mechanical
heart valve and carotid stent (n=1). No reason was
documented for one patient.
Only 41 out of the 61 patients given PCC from
February 2011 to September 2013 were included in
the PCC group for INR analysis and for comparison
with the FFP group. Twenty patients were excluded
because the reversal treatment regimen had been
modified by the treating clinicians and was different
from our protocol (n=17), and patient records were
kept by other parties and we failed to confirm the
dose given (n=3).
Their mean age was 74 years and their mean GCS on
presentation was 11. A total of 18 patients had GCS
of ≤8/were immediately intubated/planned for operation;
16 patients had GCS of >13 on presentation.
Dosage of prothrombin complex concentrates and
reversal of warfarin effect
Among the 41 patients, time of infusion of PCC was
clearly documented and INR was checked before and
within 24 hours after PCC infusion in 33 patients.
The mean pre-PCC INR was 2.81 (interquartile
range [IQR], 1.95-2.92) and the mean post-PCC INR
was 1.21 (IQR, 1.09-1.39). In 29/33 (87.9%) patients,
INR was reversed to ≤1.4 in the post-PCC INR test.
The mean time of checking the INR post-PCC was
4 hours and 15 minutes. In 11 patients, INR was
checked within 2 hours after PCC had been given.
Nine (81.8%) out of these 11 patients achieved INR
of ≤1.4 within 2 hours, with mean pre-PCC INR
being 2.43 (IQR, 2.05-2.73) and mean post-PCC INR
being 1.27 (IQR, 1.17-1.40).
Haematoma expansion
One patient underwent brain imaging in a private
hospital and, thus, the baseline haematoma volume
could not be traced. Of the 41 patients, 32 underwent
follow-up CT brain within 7 days (within 48 hours in
27 patients, within 2 to 3 days in two patients, and
between 3 and 7 days in three patients). Of these 32
patients, seven (21.9%) had haematoma expansion.
In three out of these seven patients, INR was
checked within 2 hours and corrected to ≤1.4. The
INR was not rechecked promptly in the remaining
four patients.
Thromboembolic complications
One patient had an ischaemic infarct 19 days after
ICH. This patient had underlying atrial fibrillation
and warfarin was withheld after admission.
There were, otherwise, no patients with recorded
thromboembolic events within the index admission
in the whole group of 61 patients given PCC.
Comparison between two groups receiving
either prothrombin complex concentrates or
fresh frozen plasma
Fifty-seven patients identified from January 2007 to
September 2013 had warfarin-associated ICH which
was not treated with PCC. Of these, only 44 patients
were finally included in our analysis for comparison
with the PCC group. Ten patients had not been given
any warfarin reversal agents. Three other patients
were excluded—one had started warfarin reversal
agents in another hospital and was later transferred
to our hospital days (for geographical reasons); one
was given rFVIIa; and in one patient the diagnosis of
ICH was made retrospectively, after cardiac arrest.
Mortality and functional outcome in the whole
group
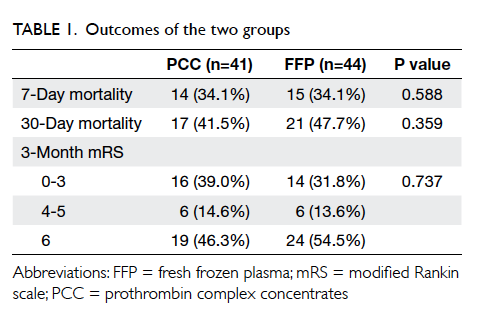
The outcomes of both groups were similar in terms
of 7-day and 30-day mortality rates and 3-month
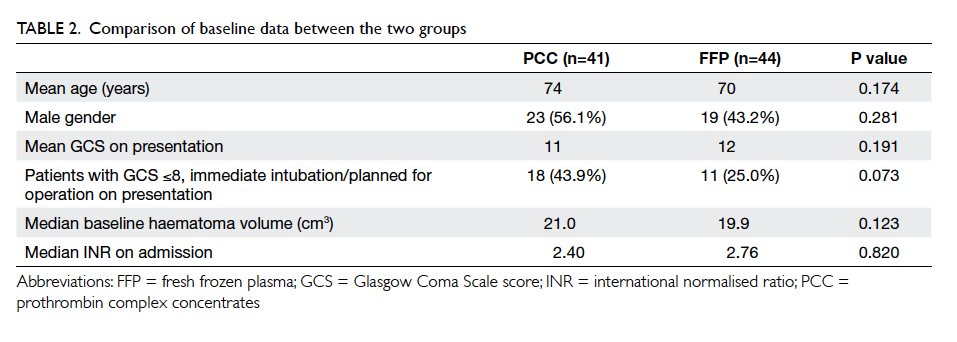
mRS (Table 1). However, there were differences in terms of proportion of target group patients. In the
PCC group, 43.9% had GCS of ≤8/intubated/already
planned for operation on admission; in contrast, only
25% from the FFP group were in this group (Table
2). Since patients with poor GCS were associated with
poor outcomes, such baseline differences probably
abolished the treatment effect of PCC over FFP.
Outcome analysis in the target group
As more than 40% of our patients had a GCS of
≤8 on presentation, and were destined to have a
poor functional outcome, we selectively compared
the outcome in patients of the target group (GCS
>8, not intubated, not initially considered for
surgery upon diagnosis of ICH) as planned. Their
baseline characteristics were similar, apart from
age which was higher in the PCC group (Table 3). The neurological deterioration rate was significantly
lower in those given PCC (n=4/23; 17.4%) versus
those given FFP (n=15/33; 45.5%; P=0.027). After
adjustment for age by logistic regression, PCC
treatment was independently associated with lower
risk of neurological deterioration (odds ratio=0.256;
95% confidence interval, 0.069-0.956; P=0.043).
Among patients who underwent follow-up CT
scan, haematoma expansion was lower in patients
given PCC for warfarin reversal (n=3/19; 15.8%)
versus those given FFP (n=10/25; 40.0%; P=0.078).
Within the target group, the 7-day and 30-day
mortality rates were lower in PCC-treated patients
(with PCC: 8.7% and 17.4%; with FFP: 12.1% and
30.3%, respectively) but the differences were not
significant (Table 3). More patients were able to
walk without assistance on follow-up at 3 months in
the PCC group versus the FFP group, without any
significant increase in dependency.
Additional analysis of all patients treated with
prothrombin complex concentrates
Of 61 patients given PCC, 20 were excluded from
target group analysis because they had not been
given PCC according to the protocol (in the form
of PCC dose variation or without concomitant
FFP). Since there might be a potential possibility of
selection bias with one third of the patients excluded
from analysis, we repeated the analysis by including
all the 61 patients. Among the 20 additional patients,
12 patients fulfilled the criteria of target group (GCS
>8, not intubated, not initially considered for surgery
upon diagnosis of ICH). Hence, the number of PCC
patients in the target group was increased from 23
to 35. On comparison between all patients given
PCC, irrespective of whether it was given according
to the protocol (n=35) and patients given FFP
(n=33), the neurological deterioration rate remained
significantly less in the PCC group (n=7/35; 20% vs
n=15/33; 45.5%; P=0.023). The haematoma expansion
rate was 21.9% (n=7/32) in the PCC group, and 40%
(n=10/25) in the FFP group (P=0.117). There were no
significant differences in the 7-day, 30-day mortality
rates, and 30-day functional outcome.
Discussion
Warfarin-associated ICH is associated with a poor
outcome and high mortality, and different studies
have demonstrated that haematoma expansion is a
causative factor. Prompt reversal of anticoagulation
has been demonstrated to decrease haematoma
expansion, and improve outcome.16 In Huttner et al’s
study,5 reversal of INR within 2 hours was associated
with less haematoma expansion, and more patients
could achieve this with PCC than with FFP. Our
study similarly showed that PCC given according
to our protocol could adequately reverse INR in a
timely manner.
However, a significant improvement in long-term
outcomes and decrease in mortality compared
to FFP treatment were not well demonstrated in our
study; these findings are also not observed in data
from published studies. This is partly due to the
difficulty in recruiting large number of patients to
demonstrate a meaningful difference in outcome.17
Furthermore, we postulate that not all patients
will benefit from rapid reversal of INR by PCC as
compared with FFP treatment. Patients with low
level of consciousness are associated with poor
outcome,4 which might not be improved no matter
what kind of reversal treatment is implemented.
In order to demonstrate improvement in clinical
outcome, studies should stratify patients according
to the neurological state on admission or depending
on whether surgery has been performed, both of
which will affect interpretation of the outcome.
On analysis of the whole PCC group
irrespective of GCS, the 30-day mortality rate was 41.5%
in our study. The 30-day mortality rate among those
with GCS of ≤8, intubated or planned for surgery on
presentation was 72.2% (n=13/18 patients), whereas
it was 17.4% (n=4/23 patients) in those in the target
group. As the mortality rate and functional outcome
are confounded by the baseline GCS level and also
by whether surgery has been done and the outcome
of the surgical procedure, we targeted our study
on the incidence of neurological deterioration for
patients with a fair-to-good admission GCS and who
had initially decided to receive conservative medical
treatment. This was the target group of patients who
was likely to benefit the most from timely treatment
in terms of decreased chances of haematoma
expansion and neurological deterioration and
subsequent poor functional outcome and death.
Our study showed that PCC treatment did decrease
the risk of deterioration significantly. And to
the best of our knowledge, it is the first study to
demonstrate that the potential benefit of PCC over
FFP is derived from patients with GCS of >8 when
treatment is started. Such benefit in the reduction
of neurological deterioration probably accounted for
the trend towards a lower mortality rate and better
functional outcome (mRS, 0-3) in the PCC group
compared with the FFP group, even though the
result was not statistically significant (which might be
related to the small sample size). Equally important
is the finding that PCC use was not associated with
a significant increase in dependency on follow-up at
3 months. This is an important finding as it suggests
that decreased mortality with PCC treatment is not
associated with an increase in the number of patients
in severely dependent state.
It is important to recognise that haematoma
expansion and deterioration can occur even in
patients with small haematomas, with the chances
of neurological deterioration being higher and the
duration more prolonged compared with those with
spontaneous ICH.3 We postulated that patients with
small haematomas or stable GCS are the ones who
would benefit most from PCC treatment as it can
reduce the chances of subsequent deterioration due
to haematoma expansion if the bleeding tendency is
not rapidly reversed. Treatment should be initiated
as soon as possible before any haematoma expansion
develops. Initiation of PCC treatment at accident and
emergency level, even before INR result is available
(as in our protocol), is probably an important factor
for improving the outcome of our patients.
The main concern in the use of PCC instead
of FFP is the associated risk of thrombosis. In our
study, one patient developed an acute ischaemic
stroke 19 days after the ICH episode. The elimination
half-lives of factor II, factor IX, and factor X within
the Prothrombinex-VF are 60 hours, 20 hours, and
30 hours, respectively.18 Hence, the ischaemic stroke
was likely related to the underlying atrial fibrillation
rather than to PCC treatment as the time interval
between its use and the ischaemic stroke was more
than five half-lives of PCC. However, in view of the
small sample size of our study, the safety of PCC
treatment with our protocol cannot be definitely
demonstrated. From other reviews, the incidence
of thrombotic events was about 1%, including deep
vein thrombosis, pulmonary embolism, myocardial
infarction, and ischaemic stroke but this risk of
thrombosis was also related to the underlying
indication of anticoagulation in the first place.11 18 19
Also, the risk may be lower with newer preparations
of PCC.18 In view of this low thrombotic risk and high
risk of haematoma expansion, the benefit of PCC
for rapid reversal of anticoagulation effect probably
overweighs the risk from its thrombogenicity,
except in the presence of contra-indications for PCC
treatment, namely evidence of active thrombosis
or disseminated intravascular coagulation. Patients
treated with PCC should be observed closely for
symptoms or signs of thrombosis, embolism,
myocardial infarction, ischaemic stroke, and
disseminated intravascular coagulation.
Limitations
The most important limitation is that it is a
retrospective comparative study of two cohorts,
instead of a randomised study. Although FFP was
the only treatment available for patients before the
introduction of PCC protocol, and PCC was given to
most patients after the protocol was implemented;
potential bias in the selection of treatment might
still occur. Some patients might be excluded from
PCC or any treatment because of their moribund
status. Also, for this study of two sequential cohorts,
there might be potential bias in that the standard
of acute care of ICH might have improved over this
7-year period, leading to better outcomes in the PCC
treatment group.
The other limitation is that the data collected
were neither complete nor standardised due to the
retrospective nature of study design. International
normalised ratios were not checked regularly at
fixed time intervals post-PCC; similarly, follow-up
CT brain scans were not performed in all
patients. Thus, the number of patients available
for analysing the adequacy of prompt INR reversal
and haematoma expansion was not large. Although
the PCC protocol recommended measuring the
INR 30 minutes after PCC administration, it was
not always performed in a busy clinical setting.
Patients who were regarded stable or too unstable
might not have undertaken follow-up CT brain to
look for haematoma expansion. Bias might also have
occurred while measuring haematoma volumes
since the raters were not blinded to the treatment
given and volumetric analysis of haematoma size was
not available. As a result, the validity in assessment
of INR reversal and haematoma expansion was
diminished. In view of the potential selection and
measurement bias inherited from the study design,
the favourable trend of the PCC treatment should
be interpreted with great caution. Similarly, the
retrospective design of the study did not allow
pre-specified investigations to reveal the potential
thrombotic complications of PCC. It was unreliable
to diagnose those complications based on review of
case notes alone, especially in such a group of ICH
patients with impaired conscious level. As a result,
the safety of PCC treatment remained unconfirmed
in this study.
Conclusions
The current study suggests that the use of PCC in
the management of warfarin-associated ICH can
promptly normalise the INR. It showed a trend of
outcome improvement in terms of reduction in
the risk of subsequent neurological deterioration,
without an increase in dependency. Whilst we await
results from an ongoing randomised study20 to
provide us with high-level evidence, PCC should be
considered the first-line reversal agent for patients
with warfarin-associated ICH after its potential
benefit has been weighed against its small but
definite risk of thromboembolism.
References
1. Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated
intracerebral hemorrhage: literature review and
expert opinion. Mayo Clin Proc 2007;82:82-92. CrossRef
2. Cucchiara B, Messe S, Sansing L, Kasner S, Lyden
P; CHANT Investigators. Hematoma growth in oral
anticoagulant related intracerebral hemorrhage. Stroke
2008;39:2993-6. CrossRef
3. Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand
J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059-64. CrossRef
4. Zubkov AY, Mandrekar JN, Claassen DO, Manno EM,
Wijdicks EF, Rabinstein AA. Predictors of outcome in
warfarin-related intracerebral hemorrhage. Arch Neurol
2008;65:1320-5. CrossRef
5. Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma
growth and outcome in treated neurocritical care patients
with intracerebral hemorrhage related to oral anticoagulant
therapy: comparison of acute treatment strategies using
vitamin K, fresh frozen plasma, and prothrombin complex
concentrates. Stroke 2006;37:1465-70. CrossRef
6. Yasaka M, Minematsu K, Naritomi H, Sakata T, Yamaguchi
T. Predisposing factors for enlargement of intracerebral
hemorrhage in patients treated with warfarin. Thromb
Haemost 2003;89:278-83.
7. Holland L, Warkentin TE, Refaai M, Crowther MA,
Johnston MA, Sarode R. Suboptimal effect of a three-factor
prothrombin complex concentrate (Profilnine-SD)
in correcting supratherapeutic international normalized
ratio due to warfarin overdose. Transfusion 2009;49:1171-7. CrossRef
8. Franchini M, Lippi G. Prothrombin complex concentrates:
an update. Blood Transfus 2010;8:149-54.
9. Baglin TP, Keeling DM, Watson HG; British Committee
for Standards in Haematology. Guidelines on oral
anticoagulation (warfarin): third edition—2005 update. Br
J Haematol 2006;132:277-85. CrossRef
10. Baker RI, Coughlin PB, Gallus AS, Harper PL, Salem
HH, Wood EM; Warfarin Reversal Consensus Group.
Warfarin reversal: consensus guidelines, on behalf of the
Australasian Society of Thrombosis and Haemostasis. Med
J Aust 2004;181:492-7.
11. Steiner T, Kaste M, Forsting M, et al. Recommendations
for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The
European Stroke Initiative Writing Committee and the
Writing Committee for the EUSI Executive Committee.
Cerebrovasc Dis 2006;22:294-316. CrossRef
12. Liumbruno G, Bennardello F, Lattanzio A, Piccoli P,
Rossetti G; Italian Society of Transfusion Medicine
and Immunohaematology (SIMTI) Working Party.
Recommendations for the use of antithrombin concentrates
and prothrombin complex concentrates. Blood Transfus
2009;7:325-34.
13. Kothari RU, Brott T, Broderick JP, et al. The ABCs of
measuring intracerebral hemorrhage volumes. Stroke
1996;27:1304-5. CrossRef
14. Hallevi H, Dar NS, Barreto AD, et al. The IVH score: a
novel tool for estimating intraventricular hemorrhage
volume: clinical and research implications. Crit Care Med
2009;37:969-74, e1.
15. Brott T, Broderick J, Kothari R, et al. Early hemorrhage
growth in patients with intracerebral hemorrhage. Stroke
1997;28:1-5. CrossRef
16. Kuwashiro T, Yasaka M, Itabashi R, et al. Effect of
prothrombin complex concentrate on hematoma
enlargement and clinical outcome in patients with
anticoagulant-associated intracerebral hemorrhage.
Cerebrovasc Dis 2011;31:170-6. CrossRef
17. Flaherty ML, Adeoye O, Sekar P, et al. The challenge
of designing a treatment trial for warfarin-associated
intracerebral hemorrhage. Stroke 2009;40:1738-42. CrossRef
18. Sørensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint
R. Clinical review: Prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care
2011;15:201. CrossRef
19. Dentali F, Marchesi C, Pierfranceschi MG, et al.
Safety of prothrombin complex concentrates for rapid
anticoagulation reversal of vitamin K antagonists. A meta-analysis.
Thromb Haemost 2011;106:429-38. CrossRef
20. Steiner T, Freiberger A, Griebe M, et al. International
normalised ratio normalisation in patients with coumarin-related
intracranial haemorrhages—the INCH trial: a
randomised controlled multicentre trial to compare
safety and preliminary efficacy of fresh frozen plasma and
prothrombin complex—study design and protocol. Int J
Stroke 2011;6:271-7. CrossRef