Hong Kong Med J 2014;20:187–93 | Number 3, June 2014 | Epub 9 May 2014
DOI: 10.12809/hkmj134069
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Characteristics and outcomes of patients with
percutaneous coronary intervention for unprotected left main
coronary artery disease: a Hong Kong experience
KY Lo, FHKCP, FHKAM (Medicine); CK Chan,
FRCP (Edin, Glasg), FHKAM (Medicine)
Division of Cardiology, Department of
Medicine and Geriatrics, United Christian Hospital, Kwun Tong,
Hong Kong
Corresponding author: Dr KY Lo (lky972@ha.org.hk)
Abstract
Objective To evaluate
the intermediate-term outcomes of patients with unprotected left
main coronary artery stenosis who were treated with percutaneous
coronary intervention in Hong Kong.
Design Historical
cohort.
Setting A regional
hospital in Hong Kong.
Patients Patients with
unprotected left main coronary artery disease undergoing
stenting with bare-metal stents or drug-eluting stents between
January 2008 and September 2011.
Main outcome measures Incidence
of restenosis and major adverse cardiac and cerebrovascular
events including cardiac death, non-fatal myocardial infarction,
stroke, and target lesion revascularisation.
Results Of the 111
patients included in the study, 86 received drug-eluting stents
and 25 received bare-metal stents. Procedural success was
achieved in 98.2% of cases. Angiographic follow-up was available
in 83.8% of cases and restenosis rate was significantly lower
with drug-eluting stents than with bare-metal stents (14.0% vs
40.0%; P=0.004). After a mean clinical follow-up of 26.1
(standard deviation, 12.6) months, the incidences of cardiac
death (5.8% vs 16.0%; P=0.191) and non-fatal myocardial
infarction (3.5% vs 8.0%; P=0.262) were similar between
drug-eluting stents and bare-metal stents. However, the risks of
target lesion revascularisation (9.3% vs 32.0%; P=0.001) and
major adverse cardiac and cerebrovascular events (19.8% vs
44.0%; P=0.004) were significantly lower with drug-eluting
stents than with bare-metal stents.
Conclusions Performing
percutaneous coronary intervention for unprotected left main
coronary artery disease was safe and feasible in selected
patients with high procedural success rate. The incidence of
major adverse cardiac and cerebrovascular events in patients
receiving drug-eluting stents remains low after
intermediate-term follow-up. Compared with bare-metal stents,
drug-eluting stents were associated with a lower need for
repeating revascularisation without increasing the risk of death
or myocardial infarction in patients with unprotected left main
coronary artery disease.
New knowledge added by this
study
- This study demonstrated that performing percutaneous coronary intervention (PCI) for unprotected left main coronary artery (ULMCA) disease in this Chinese cohort was safe and feasible in selected patients with high procedural success and good intermediate-term outcomes.
- The incidence of major adverse cardiac and cerebrovascular events in patients receiving drug-eluting stents (DES) in this cohort of patients was similar to that in other major clinical trials.
- DES was associated with a lower need for repeating revascularisation without increasing the risk of death or myocardial infarction in patients with ULMCA disease than with bare-metal stents (BMS). Our results suggested that BMS should not be encouraged due to the high incidence of restenosis and target lesion revascularisation.
- PCI in ULMCA disease can be safely performed in a centre without on-site surgical support.
Introduction
Significant unprotected left main coronary
artery (ULMCA) disease occurs in 5% to 7% of patients undergoing
coronary angiography.1
Coronary artery bypass graft (CABG) surgery has been the standard
of care for the treatment of ULMCA disease, and percutaneous
coronary intervention (PCI) is reserved for patients who are poor
surgical candidates.2
Recently, the use of drug-eluting stents (DES), together with
advance in PCI technology, has improved the outcomes of patients
undergoing PCI for ULMCA disease. The latest guidelines assign
ULMCA PCI a class IIa indication which may be considered in
patients who are at low risk for procedural complications and at
increased risk of adverse surgical outcomes.3
Because of the risk of restenosis, it is
not encouraged to use bare-metal stents (BMS) in ULMCA disease.
The situation in Hong Kong is special in this regard. The public
health care system (The Samaritan Fund) of Hong Kong does not
cover the cost of using DES in ULMCA disease. Hence, patients with
financial difficulty and who refuse to receive CABG can only
undergo PCI with BMS implantation. Moreover, like other Asian
countries, patients in Hong Kong are reluctant to have CABG,
leaving them with the option of using BMS or medical treatment
only. Because of this restraint, the proportion of patients with
ULMCA disease in Hong Kong who are treated with BMS probably
exceeds that in other developed countries.
The present study aimed to evaluate the
outcomes of patients with ULMCA stenosis who were treated with PCI
in Hong Kong.
Methods
Study population
This was a single-centre retrospective
study performed to determine the outcomes of patients who had
undergone ULMCA PCI. Between January 2008 and September 2011, 111
patients with ULMCA disease (defined as >50% stenosis) received
PCI with either DES or BMS implantation in the United Christian
Hospital, Hong Kong. The cohort included unselected consecutive
patients who presented with stable angina, acute coronary
syndrome, or cardiogenic shock. Therefore, PCI could be performed
in an elective or emergency setting (ie an all-comers basis).
Moreover, there was no on-site surgical support in our centre.
The decision of performing PCI instead of
CABG surgery was based on coronary anatomy, haemodynamic
conditions, surgical risks, and patients’ preference. Both
interventional cardiologists and cardiac surgeons were involved in
making the decision.
Unprotected left main coronary artery PCI
was performed using standard techniques. Heparin 70 to 100 units
per kg was administered before PCI. Intra-aortic balloon pump
counterpulsation, intravascular ultrasound (IVUS) or glycoprotein
IIb/IIIa inhibitors was used at the discretion of the operators.
All patients were pre-treated with 80 to 160 mg aspirin and a
loading dose of 300 to 600 mg clopidogrel or 75 mg maintenance
dose of clopidogrel at least 7 days before the procedure. After
PCI, aspirin 80 to 160 mg daily and clopidogrel 75 mg daily, for 1
month after BMS and 1 year after DES implantation, were
prescribed. For ostial and shaft left main stenosis, single stent
placement was preferred. Patients with bifurcation stenosis
underwent one of the four types of bifurcation stenting techniques
(T-stenting, T-stenting and small protrusion technique, Culotte
technique, or Crush technique) at the operators’ discretion.
Routine surveillance angiography was arranged for all patients 6
to 9 months after the index procedure, except in patients who
refused, or with high risk for coronary angiogram. Baseline
demographic, procedural, angiographic, and clinical outcome data
were collected.
Definitions
Unprotected left main coronary artery
stenosis was defined as >50% stenosis without any patent graft
to the left anterior descending artery or left circumflex artery.
Procedure was defined as successful if revascularisation was
achieved in the target lesion with <30% residual stenosis in
angiography and patient was discharged from hospital without any
of these events: death, Q-wave myocardial infarction (MI), stroke,
and target lesion revascularisation (TLR).
Follow-up was completed in June 2012.
End-points were restenosis and major adverse cardiac and
cerebrovascular events (MACCE) including cardiac death, non-fatal
MI, stroke, and TLR.
Restenosis was defined as >50% luminal
narrowing at the left main segment (stent and 5 mm proximal and
distal) which was demonstrated at the follow-up angiography,
regardless of patient symptoms.
Death was classified as cardiac or
non-cardiac. Deaths that could not be classified were considered
cardiac. Cardiac death was defined as death from any cardiac cause
(eg MI, heart failure, or arrhythmia) or sudden unexplained death
without an explanation. Non–Q-wave MI was defined as elevation of
total creatine kinase 2 times above the upper normal limit in the
absence of pathological Q wave. Target lesion revascularisation
was defined as any revascularisation performed on the treated left
main segment. Chronic kidney disease was documented if the serum
creatinine level was >200 µmol/L or was put on renal
replacement therapy. Stent thrombosis was defined as definite and
probable according to the Academic Research Consortium.4
Statistical analyses
Categorical variables reported as
percentages and comparisons between groups were based on the Chi
squared test or Fisher’s exact test. Continuous variables were
reported as mean ± standard deviation, and differences were
assessed with the independent sample t test or
Mann-Whitney test.
Cumulative event curves were calculated by
the Kaplan-Meier method and compared by the log-rank test. A P
value of <0.05 was considered statistically significant.
Statistical analyses were performed with the use of the
Statistical Package for the Social Sciences (Windows version 15.0;
SPSS Inc, Chicago [IL], US).
Results
Patient characteristics
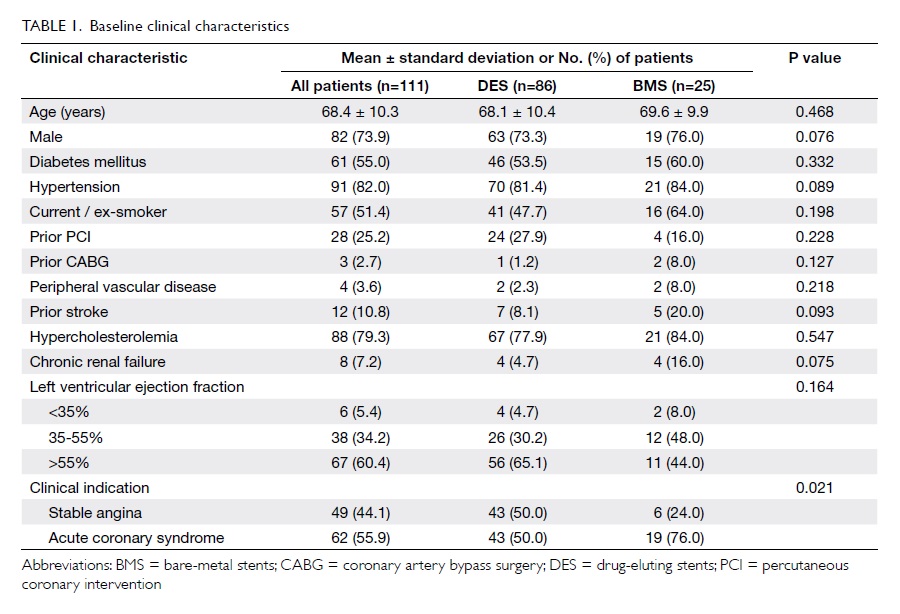
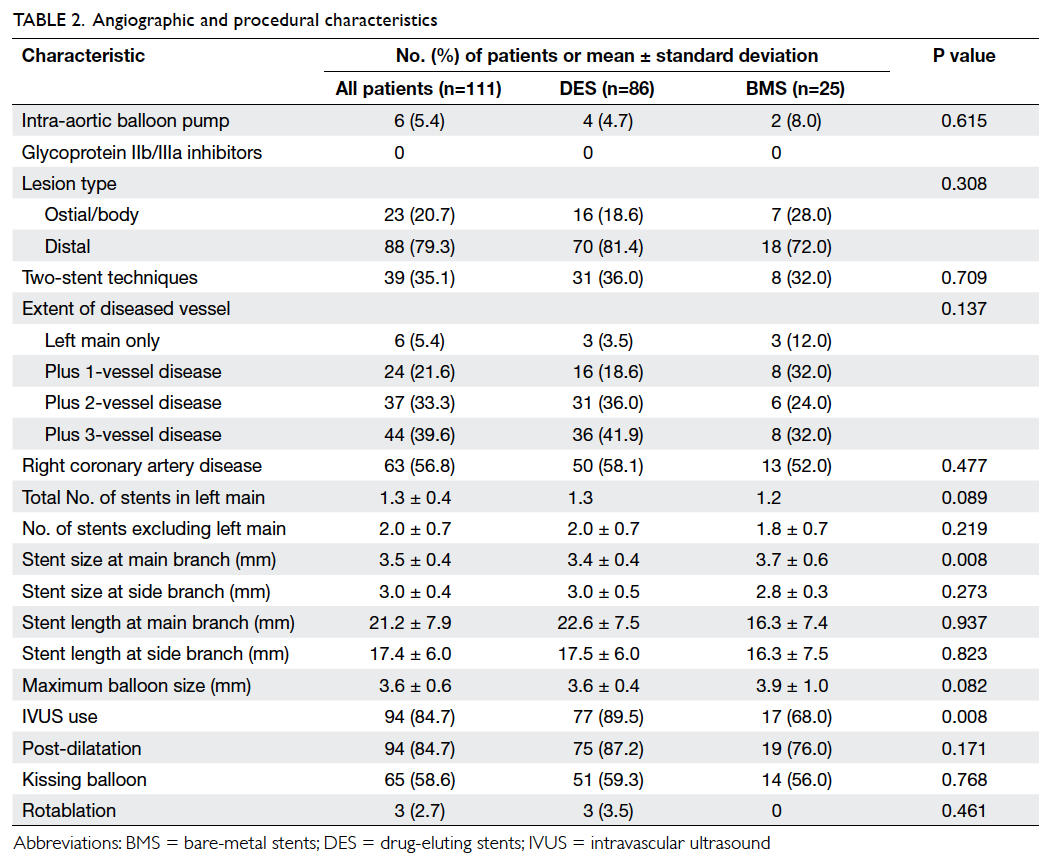
Baseline clinical, and angiographic and
procedural characteristics of the 111 patients are summarised in Table 1and Table 2, respectively.
Overall, 86 (77.5%) patients were treated
with DES, and 25 (22.5%) received BMS. The two groups shared
similar clinical and angiographic characteristics. More than 90%
of patients had left ventricular ejection fraction of ≥35%. The
majority of patients had distal left main disease (81.4% in DES
group and 72.0% in BMS group). Only a minority of patients (5.4%)
had isolated left main disease, whereas 72.9% had left main and at
least two-vessel disease. A high rate of IVUS use was observed in
the cohort (84.7%). Final kissing balloon dilatation was performed
in >50% of the patients and in all patients with two-stent
approach. Other adjuvant PCI devices such as rotational
atherectomy were rarely required in this cohort.
Of the 86 patients who received DES at the
left main segment, 24 (27.9%) received first-generation DES, 56
(65.1%) received second-generation DES, and six (7.0%) received
both types.
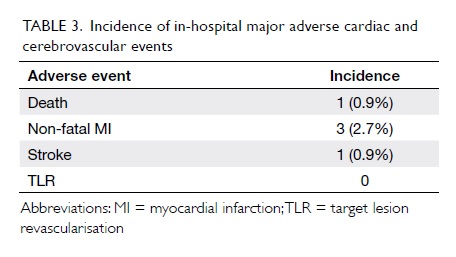
Outcomes
Procedural success was achieved in 109/111
(98.2%) cases. There was one death (0.9%) and one stroke (0.9%)
but there was no Q-wave MI, stent thrombosis, or urgent repeat
revascularisation events during hospitalisation (Table
3).
The mean duration of clinical follow-up was
26.1 ± 12.6 months. Table 4 depicts the incidence of adverse
outcomes in all patients at the end of follow-up. There was no
significant difference between the DES and BMS groups in the
cumulative incidences of cardiac death (5.8% for DES vs 16.0% for
BMS; P=0.191) or non-fatal MI (3.5% vs 8.0%; P=0.262). Compared
with BMS, use of DES was associated with significantly lower risks
of TLR (9.3% vs 32.0%; P=0.001) and MACCE (19.8% vs 44.0%;
P=0.004) [Fig]. Target lesion revascularisation was
ischaemia-driven in 4/16 (25%) patients; in the remaining 12/16
(75%) patients, TLR was driven by restenosis identified at
surveillance angiography after the index procedure. Therefore, the
crude rate of ischaemia-driven TLR was only 4/111 (3.6%) in the
overall cohort. The mean timing of TLR was 7.6 ± 4.3 months
(range, 2-16 months) after the index procedure.
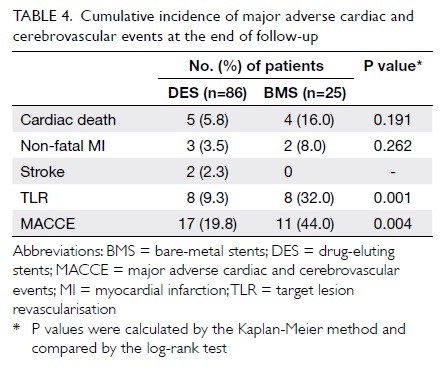
Table 4. Cumulative incidence of major adverse cardiac and cerebrovascular events at the end of follow-up
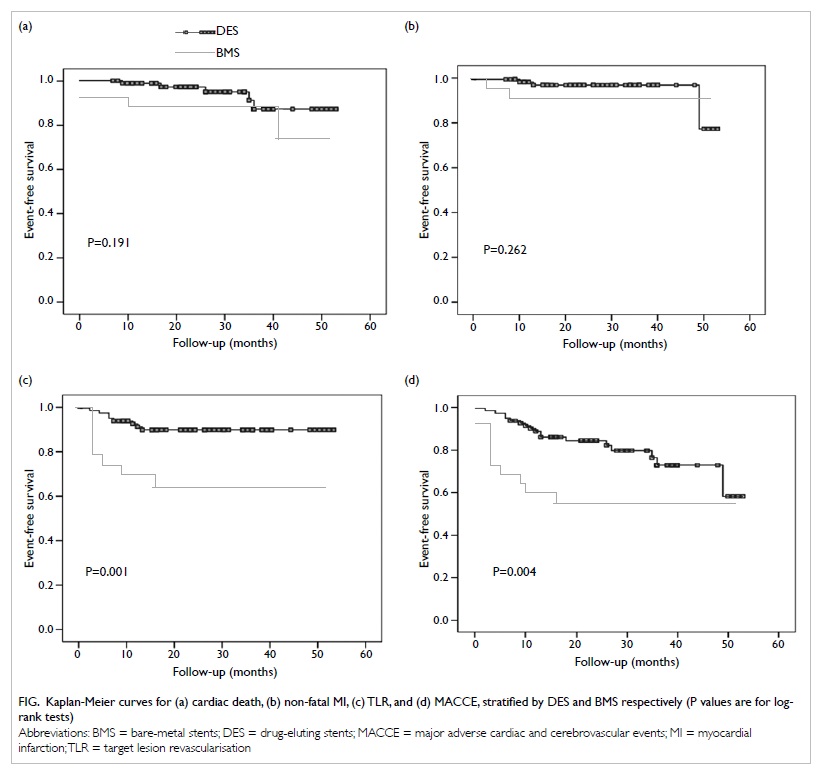
Figure. Kaplan-Meier curves for (a) cardiac death, (b) non-fatal MI, (c) TLR, and (d) MACCE, stratified by DES and BMS respectively (P values are for logrank tests)
Of 111 cases, 93 (83.8%) underwent routine
surveillance angiography 6 to 9 months after PCI; binary
restenosis occurred in 22/111 (20%) cases. Restenosis occurred
predominantly in patients with distal left main coronary artery
disease (19/22 [86%]); and more than half of them (12/22 [55%])
had isolated focal restenosis involving the ostium of the left
circumflex artery only. Restenosis occurred less frequently with
DES than with BMS (12/86 [14.0%] vs 10/25 [40.0%]; P=0.004).
For stent thrombosis, the event rate was
extremely low across the whole cohort. One patient receiving BMS
implantation developed subacute stent thrombosis after hospital
discharge (which resulted in sudden cardiac death). There was no
stent thrombosis of any forms in the DES group.
Discussion
The principal findings of the present study
were: (1) performing PCI for ULMCA disease was safe and feasible
in selected patients with high procedural success rate (98.2%);
(2) after an intermediate-term follow-up of 26.1 months, the
incidence of MACCE in patients receiving DES implantation was
similar to that reported in recent major international clinical
trials including the SYNTAX trial5;
(3) compared with BMS, the use of DES was associated with a lower
risk of restenosis and repeat revascularisation without an
increased risk of death or MI.
Historically, CABG has been regarded as the
gold standard of treatment for ULMCA disease. Clinical outcomes
after PCI for ULMCA stenosis have been shown to vary widely,
according to patients’ clinical and angiographic features.6 7 The
high procedural success rate in our study further confirms the
technical feasibility of treating ULMCA lesions with the current
PCI techniques in the absence of on-site surgical support.
Promising results were reported from
randomised trials comparing first-generation DES versus CABG.5 8 9 In the SYNTAX trial,5
patients were stratified according to the presence of ULMCA
disease and randomised to CABG (n=348) or PCI with
paclitaxel-eluting stents (n=357). In the ULMCA subgroups, MACCE
at 12 months was comparable between patients treated with PCI and
CABG. Moreover, although the rate of repeat revascularisation
among patients with ULMCA disease was significantly higher in the
PCI subgroup, this result was offset by a significantly higher
rate of stroke in the CABG subgroup.
The SYNTAX trial5
included patients with heterogeneous angiographic characteristics
in the left main subgroup (13% with isolated left main coronary
artery disease, 20% with left main plus single-vessel disease, 31%
with two-vessel disease, and 37% with triple-vessel disease).
Although calculation of the SYNTAX score was not incorporated in
routine clinical practice at the time of our study, our cohort
demonstrated similar heterogeneity and complexity (Table 2).
We report an intermediate-term outcome
(mean follow-up of approximately 26 months) for patients with
ULMCA PCI, and our results were comparable with those of the
SYNTAX trial.5 At 2 years,
the SYNTAX trial5 reported
a MACCE rate of 22.9% in the left main subgroup (including death
from any causes, MI, stroke, or repeat revascularisation), which
was comparable with the incidence of 19.8% reported in our study.
The incidence of TLR in the subgroup of DES
in our registry (9.3%) might be lower than that reported in the
SYNTAX trial5 at 2 years
(any revascularisation, 17.3%) and it might be due to inclusion of
second-generation DES in two thirds of the patients treated with
DES in our registry. The higher rate of IVUS use for optimisation
(approximately 90% of cases using DES in our cohort) might also be
another reason. One of the main limitations of the SYNTAX trial
was thought to be the lack of IVUS use for ULMCA disease in the
PCI group. Clinical trials10
have shown that patients whose coronary interventions are guided
by IVUS have larger post-procedure stent areas and significant
reductions in TLR than those undergoing angiography-guided PCI
only. Registry data have also shown a trend towards reduced
mortality in IVUS-guided ULMCA PCI.11
It is worth considering that SYNTAX did not
have an ‘all-comers’ design, where patients with acute coronary
syndrome and cardiogenic shock were excluded. Our registry did
have an ‘all-comers’ design, by including patients presenting with
stable angina, acute coronary syndrome, ST-elevation and non–ST
elevation MI, as well as cardiogenic shock. This might reflect a
more ‘real-world’ situation in daily clinical practice. Despite
the inclusion of patients with higher clinical risk, the incidence
of events remained low in our study during the index hospital
admission and upon medium-term follow-up.
In the BMS subgroup, we reported a high
incidence of restenosis (40%) and TLR (32%). To date, no
randomised controlled trials have been performed using BMS in
ULMCA PCI. The longest follow-up available in the literature was
from the ASAN-MAIN (ASAN Medical Center–Left MAIN
Revascularization) Registry (n=350: BMS, n=100; CABG, n=250),12 which also reported a high rate of TLR
(24.9%) after long-term follow-up. Although the incidence of
restenosis and TLR might be over-represented due to the use of
routine surveillance angiography in our study, the results suggest
that the use of BMS was not favoured.
As mentioned, the situation in Hong Kong is
unique in that the public health care system does not cover the
cost of using DES in ULMCA disease. Patients with financial
difficulty can only choose PCI with BMS or CABG. Because of this
restraint, the proportion of patients with ULMCA disease in Hong
Kong treated with BMS probably exceeds that in other developed
countries. In our opinion, a review of this health care policy is
necessary.
In our cohort, the rate of cardiac deaths
in the BMS group was relatively high (16.0% in BMS vs 5.8% in
DES). While this could be a finding by chance, it could be
attributed to a multitude of reasons. Compared with the DES group,
a higher proportion of patients presented with acute coronary
syndrome including cardiogenic shock in the BMS group (Table 1).
Moreover, there was a higher proportion of patients with chronic
renal failure or prior stroke in the BMS group (Table 1). Such
differences might explain the relatively high cardiac mortality
rates in the BMS group. Another postulation is that patients who
received BMS implantation may have come from a lower
socio-economic class, which might have an impact on their health
status and outcome.
The role of routine surveillance
angiography remains unclear and controversial. Repeat angiography
is suggested because patients with left main restenosis are
considered to be at high risk for adverse events. However,
angiography is unable to predict when a patient might be prone to
stent thrombosis, and angiography might be associated with a
non-negligible risk in patients who have undergone left main
stenting.13 Therefore, the
2009 focused update does not recommend routine angiographic
follow-up after ULMCA stenting.14
Our result is in line with the guideline as the angiographic
restenosis rate in the DES group was low. This would have been
even lower had a clinically driven approach been used. Given the
low event rate in our cohort, we also recommend that routine
surveillance angiography is not necessary and patients can be
followed up clinically.
An interesting point is that the risk of
stent thrombosis was extremely low (<1%) given the standard
prescription of 1-year dual antiplatelet therapy with aspirin and
clopidogrel in this group of high-risk patients with multiple
complex stenting. No laboratory or genetic assessment was
performed on the degree of platelet function inhibition.
The present study had several limitations.
Firstly, it was a single-centre non-randomised retrospective
study, which might have significantly affected the results due to
unmeasured confounders, procedure bias, or detection bias.
Secondly, angiographic results were based on visual angiographic
or IVUS assessment and a standardised core laboratory anatomical
examination was not performed. Thirdly, incomplete angiographic
follow-up might underestimate the incidence of restenosis.
Finally, this study included high-risk patients with complex
coronary anatomy who underwent PCI (including patients who refused
bypass surgery); these patients were prone to poor clinical
outcomes. Therefore, these results might not be generalised to all
populations with ULMCA stenosis, especially those with
low-to-intermediate SYNTAX score.
Conclusions
These are the largest available data on
ULMCA PCI in Hong Kong. Performing PCI for ULMCA disease was safe
and feasible in selected patients with high procedural success.
Despite the inclusion of high-risk patients, the incidence of
MACCE after intermediate-term follow-up in patients receiving DES
implantation was similar to that reported in major clinical
trials. Compared with BMS, DES was associated with a reduced need
for repeat revascularisation without increasing the risk of death
or MI for patients with ULMCA disease. Our result suggest that BMS
should not be encouraged due to the high incidence of restenosis
and TLR.
Declaration
The authors report no financial
relationships or conflicts of interest regarding the content
herein.
Acknowledgements
The authors wish to thank Dr CY Mui and Dr
TK Lau for their assistance in data collection.
References
1. DeMots H, Rösch J, McAnulty JH,
Rahimtoola SH. Left main coronary artery disease. Cardiovasc Clin
1977;8:201-11.
2. Eagle KA, Guyton RA, Davidoff R,
et al. ACC/AHA 2004 guideline update for coronary artery bypass
graft surgery: a report of the American College of
Cardiology/American Heart Association Task Force on Practice
Guidelines (Committee to Update the 1999 Guidelines for Coronary
Artery Bypass Graft Surgery). Circulation 2004;110:1168-76. CrossRef
3. Levine GN, Bates ER, Blankenship
JC, et al. 2011 ACCF/AHA/ SCAI guideline for percutaneous coronary
intervention. A report of the American College of Cardiology
Foundation/ American Heart Association Task Force on Practice
Guidelines and the Society for Cardiovascular Angiography and
Interventions. J Am Coll Cardiol 2011;58:e44-122. CrossRef
4. Cutlip DE, Windecker S, Mehran
R, et al. Clinical end points in coronary stent trials: a case for
standardized definitions. Circulation 2007;115:2344-51. CrossRef
5. Serruys PW, Morice MC, Kappetein
AP, et al. Percutaneous coronary intervention versus
coronary-artery bypass grafting for severe coronary artery
disease. N Engl J Med 2009;360:961-72. CrossRef
6. Park SJ, Kim YH, Lee BK, et al.
Sirolimus-eluting stent implantation for unprotected left main
coronary artery stenosis: comparison with bare metal stent
implantation. J Am Coll Cardiol 2005;45:351-6. CrossRef
7. Price MJ, Cristea E, Sawhney N,
et al. Serial angiographic follow-up of sirolimus-eluting stents
for unprotected left main coronary artery revascularization. J Am
Coll Cardiol 2006;47:871-7. CrossRef
8. Park SJ, Kim YH, Park DW, et al.
Randomized trial of stents versus bypass surgery for left main
coronary artery disease. N Engl J Med 2011;364:1718-27. CrossRef
9. Boudriot E, Thiele H, Walther T,
et al. Randomized comparison of percutaneous coronary intervention
with sirolimus-eluting stents versus coronary artery bypass
grafting in unprotected left main stem stenosis. J Am Coll Cardiol
2011;57:538-45. CrossRef
10. Hong MK, Mintz GS, Lee CW, et
al. Intravascular ultrasound predictors of angiographic restenosis
after sirolimus-eluting stent implantation. Eur Heart J
2006;27:1305-10. CrossRef
11. Park SJ, Kim YH, Park DW, et
al. Impact of intravascular ultrasound guidance on long-term
mortality in stenting for unprotected left main coronary artery
stenosis. Circ interventions 2009;2:167-77.
12. Park DW, Kim YH, Yun SC, et
al. Long-term outcomes after stenting versus coronary artery
bypass grafting for unprotected left main coronary artery disease.
J Am Coll Cardiol 2010;56:1366-75. CrossRef
13. Lee MS, Kapoor N, Jamal F, et
al. Comparison of coronary artery bypass surgery with percutaneous
coronary intervention with drug-eluting stents for unprotected
left main coronary artery disease. J Am Coll Cardiol
2006;47:864-70. CrossRef
14. Kushner FG, Hand M, Smith SC
Jr, et al. 2009 focused updates: ACC/AHA guidelines for the
management of patients with ST-elevation myocardial infarction
(updating the 2004 guideline and 2007 focused update) and ACC/
AHA/SCAI guidelines on percutaneous coronary intervention
(updating the 2005 guideline and 2007 focused update). J Am Coll
Cardiol 2009;54:2205-41. CrossRef