Hong Kong Med J 2014;20:102–6 | Number 2, April 2014
DOI: 10.12809/hkmj134065
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Live birth rate, multiple pregnancy rate, and obstetric outcomes of elective single and double embryo transfers: Hong Kong experience
Joyce Chai, FHKAM (Obstetrics and Gynaecology);
Tracy WY Yeung, FHKAM (Obstetrics and Gynaecology);
Vivian CY Lee, FHKAM (Obstetrics and Gynaecology);
Raymond HW Li, FHKAM (Obstetrics and Gynaecology);
Estella YL Lau, PhD;
William SB Yeung, PhD;
PC Ho, MD, FHKAM (Obstetrics and Gynaecology);
Ernest HY Ng, MD, FHKAM (Obstetrics and Gynaecology)
Department of Obstetrics and Gynaecology, Queen Mary Hospital, The
University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr J Chai (jchai@hkucc.hku.hk)
Abstract
Objective: To compare the live
birth rate, multiple pregnancy rate, and obstetric
outcomes of elective single and double embryo
transfers.
Design: Case series with internal comparisons.
Setting: University affiliated hospital, Hong Kong.
Participants: Between October 2009 and December
2011, 206 women underwent their first in-vitro
fertilisation cycle. Elective single embryo transfer
was offered to women who were aged 35 years or
below, and had endometrial thickness of 8 mm or
more and at least two embryos of good quality.
Main outcome measures: Live birth rate, multiple
birth rate, and obstetric outcomes.
Results: Among the 206 eligible women, 74
underwent an elective single embryo transfer and
132 a double embryo transfer. The live birth rate was
comparable in the two groups, being 39.2% in the
elective single embryo transfer group and 43.2% in
the double embryo transfer group, while the multiple
pregnancy rate was significantly lower in the elective
single embryo transfer group than the double embryo
transfer group (6.9% vs 40.4%; P<0.001). Gestational
ages and birth weights were comparable in the two groups. There was no significant difference between
the two groups with respect to the rate of preterm
delivery and antenatal complications (27.6% vs
43.9%, respectively; P>0.05).
Conclusion: In this selected population, an elective
single embryo transfer policy decreases the multiple
pregnancy rate without compromising the live birth
rate. The non-significant difference in antenatal
complications may be related to the small sample
size.
New knowledge added by this
study
- Elective single embryo transfer decreased the multiple pregnancy rate without compromising the live birth rate in women with a good prognosis undergoing in-vitro fertilisation.
- Elective single embryo transfer should be offered to women with a good prognosis and the care provider should promote this policy through education.
Introduction
In-vitro fertilisation (IVF) treatment is an effective
treatment for various causes of infertility and
involves development of multiple follicles after
ovarian stimulation, oocyte retrieval, and embryo
transfer after fertilisation. Historically, multiple
embryos were transferred to compensate for low
rates of implantation for individual embryos as well
as to achieve higher pregnancy rates. Consequently,
IVF carried a high risk of multiple pregnancy and its
associated adverse effects on mothers and children.1 In 2003 the Chairman of the European Society of
Human Reproduction and Embryology (ESHRE) commented that
assisted reproduction techniques should result in the
birth of one healthy child and that a twin pregnancy
should be regarded as a complication.2
In January 2013, the “Code of Practice on
Reproductive Technology & Embryo Research”
issued by the Council on Human Reproductive
Technology of Hong Kong stipulated that no more
than three embryos should be placed in a woman
in any one cycle.3 In August 2001, the Human Fertilisation and Embryology Authority in the UK
recommended reducing the number of embryos that
should be transferred in a single IVF treatment cycle
from three to two.4
In 2001, ESHRE recommended elective single
embryo transfer (eSET) for women aged under
34 years at the time of their first attempt, as soon
as they had obtained a top-quality embryo.5 In
2008, the British Fertility Society, in conjunction
with the Association of Clinical Embryologists,
introduced guidelines for eSET in the UK that aimed
to reduce IVF multiple pregnancy rates to less than
10%.6 Meta-analyses have shown that in a selected
population, compared with double embryo transfer
(DET), eSET could reduce multiple pregnancy rates
significantly, without compromising cumulative
pregnancy rates.7 8
Our centre offered eSET to eligible women in
order to reduce the multiple pregnancy rate. The
aim of this study was to compare the live birth rate,
multiple pregnancy rate, and obstetric outcomes
after eSET and DET in mothers having their first IVF/intra-cytoplasmic sperm injection (ICSI) attempt.
Methods
This was a retrospective study carried out at the
Centre of Assisted Reproduction and Embryology,
Queen Mary Hospital, The University of Hong Kong,
Hong Kong. Clinical details of all treatment cycles were prospectively entered into a computerised
database, and checked for correctness and
completeness on a regular basis. For this study, data
were retrieved for analysis and ethics committee
approval was deemed not necessary for retrospective
analysis of data.
Patients
In our programme, a maximum of two embryos were
replaced, irrespective of the woman’s age. Women
were eligible for eSET if they were ≤35 years of age
at the time of the embryo transfer, were undergoing
their first IVF cycle, had an endometrial thickness of
≥8 mm, and had at least two good-quality embryos
available for transfer or freezing. Good-quality
embryos were defined by their morphological
features and cleavage rate, and included embryos
with less than 25% fragmentation and four cells at
day 2. Eligible patients were individually counselled
about eSET. Women who opted for eSET would have
one embryo replaced (eSET group), while those who
opted for DET had two embryos transferred (DET
group).
Ovarian stimulation and in-vitro fertilisation/intra-cytoplasmic sperm injection procedures
All women were treated either with the long
gonadotropin-releasing hormone (GnRH) agonist
protocol or the GnRH antagonist protocol for
pituitary down-regulation. The details of the long
protocol for the ovarian stimulation regimen,
handling of gametes, as well as standard insemination
and ICSI were as previously described.9 In short,
women received buserelin (Suprecur; Hoechst,
Frankfurt, Germany) nasal spray 150 μg 4 times a
day starting from the mid-luteal phase of the cycle
preceding the treatment cycle, followed by human
menopausal gonadotropins (hMG) or recombinant
follicle-stimulating hormone (FSH) for ovarian
stimulation after return of a period. In the GnRH
antagonist protocol, after confirming a basal serum
oestradiol level, ovarian stimulation was started
with either hMG or recombinant FSH. Ganirelix
(NV Organon; Swords Co, Dublin, Ireland) 250 μg
was started from the sixth day of stimulation. The
starting dose of gonadotropin was based on the
baseline antral follicle count.
Transvaginal ultrasonography was used to
monitor the ovarian response. When the mean
diameter of the leading follicle reached 18 mm and
there were at least three follicles reaching a mean
diameter of 16 mm or more, human chorionic
gonadotropin (hCG; Pregnyl; Organon, Oss,
The Netherlands) 5000 or 10 000 units or Ovidrel
(Merck Serono, Modugno, Italy) 250 μg was given
and oocytes were collected about 36 hours later.
Fertilisation was carried out in vitro either by conventional insemination or ICSI depending on
semen parameters. Women were allowed to have
replacement of at most two embryos 2 days after
oocyte retrieval. A progesterone pessary (Endometrin
100 mg twice per day; Ferring Pharmaceuticals,
Parsippany [NJ], US) was administered from the
day of embryo transfer for 2 weeks to enable luteal
support. Pregnancies were confirmed by positive
urine hCG tests and transvaginal ultrasonographic
evidence of a gestational sac.
Collection of clinical information
Clinical information including age, body mass index,
basal serum levels of FSH, and baseline antral follicle
counts were collected. During IVF treatment, such
data included days of stimulation, total dosage of
gonadotropin, oestradiol level on day of hCG,
number of oocytes retrieved, number of available
embryos, number of good-quality embryos, as well
as pregnancy and miscarriage rates.
Clinically, pregnancy was defined as the
presence of a gestational sac by ultrasonography,
whereas the miscarriage rate per clinical pregnancy
was defined as the proportion of patients whose
pregnancy failed to develop before 20 weeks of
gestation. Pregnancy outcome was collected from
all pregnant women by a postal questionnaire or by
phone. Live birth was defined as the delivery of a
fetus with signs of life after 24 completed weeks of
gestational age, and the multiple pregnancy rate was
calculated as the number of multiple pregnancies
divided by the number of clinical pregnancies,
expressed as a percentage. Obstetric outcomes
including antenatal complications, gestational age
at delivery, mode of delivery, and birth weight were
also recorded.
Statistical analysis
The primary outcome measure was the live birth
rate and secondary outcomes included the multiple
pregnancy rate and obstetric outcomes. Statistical
analysis for the comparison of mean values was
performed using Mann-Whitney and Student’s t
tests, as appropriate. The Chi squared and Fisher’s
exact tests were used to compare categorical
variables. Statistical analysis was carried out using
the Statistical Package for the Social Sciences
(Windows version 20.0; SPSS Inc, Chicago [IL],
US). A two-tailed P value of <0.05 was considered
statistically significant.
Results
In all, 206 women undergoing their first IVF cycle
from October 2009 to December 2011 met the
inclusion criteria. A total of 74 women chose eSET
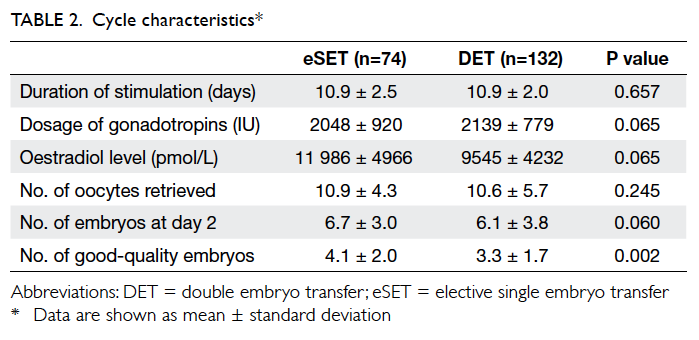
and 132 chose DET. Patient and cycle characteristics
are shown in Tables 1 and 2, respectively. Women who opted for eSET were significantly younger than
those opting for DET, and had a significantly higher
proportion of good-quality embryos than those in
the DET group.
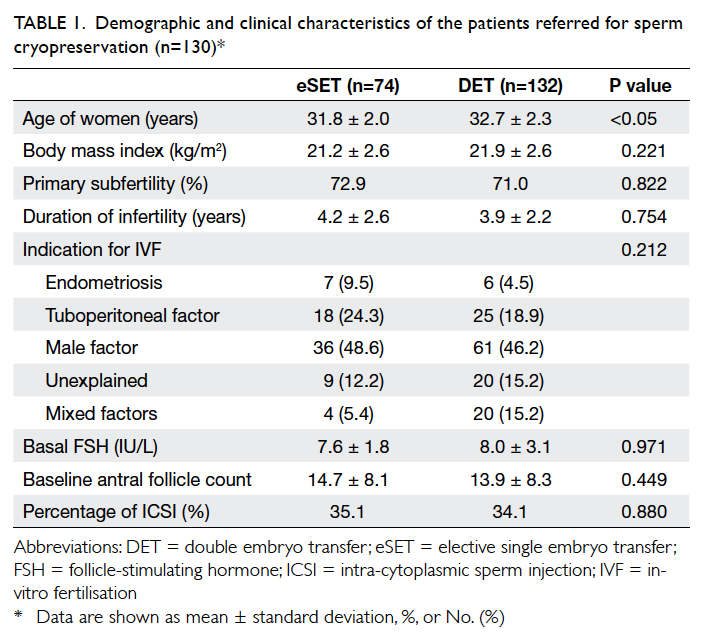
Table 1. Demographic and clinical characteristics of the patients referred for sperm cryopreservation (n=130)*
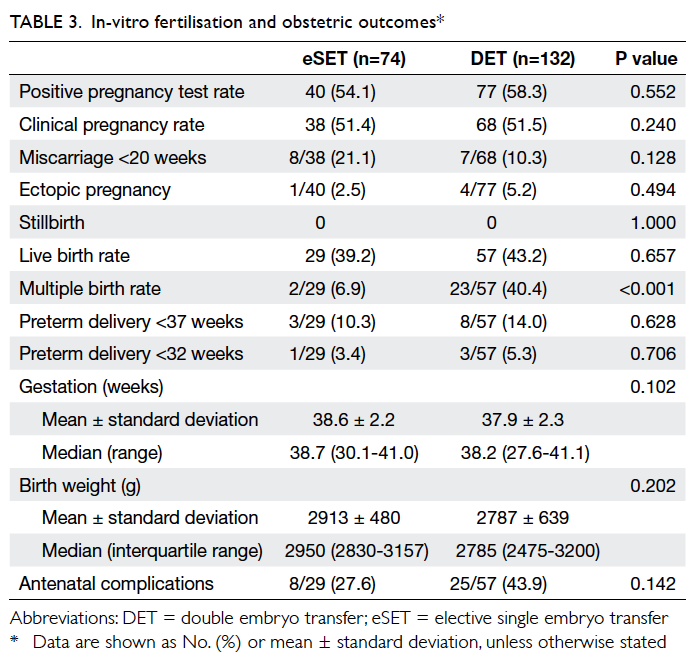
The IVF and obstetric outcomes are shown in
Table 3. Among women with eSET, 40 (54.1%) had
a positive pregnancy test; two were biochemical
pregnancies, eight miscarried, and one was an ectopic
pregnancy. There was one pair of monozygotic and
one pair of dizygotic twins in the eSET group. In
women having DET, the positive pregnancy test rate
was 58.3% (n=77/132); there were nine biochemical
pregnancies, seven miscarriages, and four ectopic
pregnancies. In the DET group, the multiple
pregnancy rate was 40.4%, which was significantly higher than that
in the eSET group (P<0.001). There were two sets
of triplets, of which one underwent fetal reduction
to a singleton and the other had fetal reduction to
twins. One woman in the eSET group and four in the DET group were lost to follow-up for their
obstetric outcomes. Overall, the live birth rate was
comparable in the eSET and DET groups (39.2% vs
43.2%, respectively).
The mean gestational age at birth and
the median birth weight were not significantly
different in the eSET group compared with the
DET group (38.6 ± 2.2 vs 37.9 ± 2.3 weeks and
2950 [interquartile range, 2830-3157] g vs 2785
[2475-3200] g, respectively). The preterm delivery
rate (defined as delivery at <37 weeks) and the
frequencies of antenatal complications (including
gestational diabetes, gestational hypertension, pre-eclampsia,
and placenta praevia) were higher in the
DET group, although the difference did not reach
statistical significance.
Discussion
The risk of multiple pregnancy has been a concern
in IVF/ICSI as it is associated with adverse maternal
and neonatal outcomes.1 This is the first study
reporting live birth rates and obstetric outcomes
after eSET and DET in a selected population in
Hong Kong. Our study confirms recent literature
findings,7 8 by showing that eSET can significantly
reduce the multiple pregnancy rate without adversely
affecting the live birth rate in young women with
good ovarian function. No triplets were observed
in the eSET group, but rather unexpectedly it did
contain two pairs of twins; one was monozygotic and one dizygotic. Dizygotic twin pregnancy following
a single embryo transfer was a rare event, and
suggestive of a spontaneous pregnancy occurring
concurrently with one due to IVF.10 The multiple
pregnancy rate of 40.4% in the DET group and the
live birth rates in our study (39.2% and 43.2% in the
eSET and DET groups, respectively) were similar to
or higher than those previously reported.7 11 12 13 14
Our study showed that the obstetric outcomes
were not significantly different in the two groups.
Antenatal complications were more common in the
DET group (43.9% vs 27.6% in eSET group), although
the difference did not reach statistical significance
(P=0.142). Regrettably, data on the Apgar score,
neonatal intensive care unit admissions, and
perinatal mortality were not available. A recent meta-analysis
by Grady et al15 showed that eSET babies
were associated with decreased risks of preterm
birth and low birth weight than those involving DET.
Moreover, eSET singletons had a higher birth weight
and lower preterm birth rate than DET singletons,
which was postulated to be related to the vanishing
twin.16 Our study failed to demonstrate the difference
but this could be attributed to the small sample size.
Our study was limited by its retrospective
nature and small sample size. Also, women having
eSET were significantly younger than those having
DET, which might lead to possible confounding.
The younger mean age in the eSET group could
explain the higher number of good-quality embryos
available for transfer, which might have an impact
on the cumulative pregnancy rate. The cumulative
pregnancy rate was not always included as many
women still had frozen embryos, but this would be an
important aspect to look into in the future. Another
bias was that women were allowed to choose between
one or two embryos to transfer, instead of allocation
by randomisation. Nonetheless, it reflected the
actual situation in our centre. Blastocyst transfer is
not routinely performed in out unit, because of the
possible increased risk of congenital abnormalities
and preterm labour,17 18 although the pregnancy and
live birth rates of the fresh cycle may be higher than
those following early cleavage stage transfer.19
The eSET policy is increasingly being applied
and in a country like Belgium, the law requires eSET
for all patients aged under 36 years during their
first two IVF attempts.20 In Hong Kong, eSET is not
imposed and suitable women were given the choice
of eSET and DET with detailed counselling. From our
data, only a third of the women chose eSET, which
suggests that such women are still resistant to eSET.
Child et al21 found that 41% of women having assisted
reproductive technology were actually inclined to
prefer a twin pregnancy, and some women waiting
for IVF treatment viewed severe child disability
outcomes more desirable than having no child at
all.22 This barrier might be overcome by providing educational material to women so as to improve
their knowledge on outcomes and risks of multiple
pregnancies.23 The feasibility of eSET also relies on
improving outcomes with cryopreserved embryos
and the technique on vitrification. Information from
the present study may also improve the uptake of
eSET in the unit.
Our study confirms that when compared
with DET, eSET can reduce the rate of multiple
pregnancies without compromising the live birth
rate in the fresh cycle. Elective SET should be offered
to patients with a good prognosis and IVF centres
should promote it, whenever appropriate, through
provider and patient education.
Acknowledgements
The authors would like to thank Mr TM Cheung for
data collection.
Declaration
No conflicts of interest were declared by the authors.
References
1. Pinborg A. IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update 2005;11:575-93. CrossRef
2. Land JA, Evers JL. Risks and complications in assisted reproduction techniques: report of an ESHRE consensus meeting. Hum Reprod 2003;18:455-7. CrossRef
3. Council on Human Reproductive Technology, Hong Kong. Code of Practice on Reproductive Technology & Embryo Research. Available from: http://www.chrt.org.hk/english/service/service_cod.html. Accessed Apr 2013.
4. HFEA reduces maximum number of embryos transferred in single IVF treatment from three to two [press release]. Human Fertilisation and Embryology Authority; 2001 Aug 8.
5. Prevention of twin pregnancies after IVF/ICSI by single embryo transfer. ESHRE Campus Course Report. Hum Reprod 2001;16:790-800.
6. Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A; BFS and ACE. Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb) 2008;11:131-46. CrossRef
7. McLernon DJ, Harrild K, Bergh C, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ 2010;341:c6945. CrossRef
8. Pandian Z, Bhattacharya S, Ozturk O. Number of embryos for transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database Syst Rev 2009:CD003416.
9. Ng EH, Yeung WS, Lau EY, So WW, Ho PC. High serum oestradiol levels in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent FET cycles. Hum Reprod 2000;15:250-5. CrossRef
10. Van der Hoorn ML, Helmerhorst F, Claas F, Scherjon S. Dizygotic twin pregnancy after transfer of one embryo. Fertil Steril 2011;95:805.e1-3.
11. Gremeau AS, Brugnon F, Bouraoui Z, Pekrishvili R, Janny L, Pouly JL. Outcome and feasibility of elective single embryo transfer (eSET) policy for the first and second IVF/ICSI attempts. Eur J Obstet Gynecol Reprod Biol 2012;160:45-50. CrossRef
12. Fauque P, Jouannet P, Davy C, et al. Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers. Fertil Steril 2010;94:927-35. CrossRef
13. Thurin A, Hausken J, Hillensjo T, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med 2004;351:2392-402. CrossRef
14. Council on Human Reproductive Technology, Hong Kong. Reports and statistics. CHRT website: www.chrt.org.hk/english/publications/publications_rep.html. Accessed Apr 2013.
15. Grady R, Alavi N, Vale R, et al. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. Fertil Steril 2012;97:324-31. CrossRef
16. De Sutter P, Delbaere I, Gerris J, et al. Birthweight of singletons after assisted reproduction is higher after single- than after double-embryo transfer. Hum Reprod 2006;21:2633-7. CrossRef
17. Källén B, Finnström O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril 2010;94:1680-3. CrossRef
18. Dar S, Librach CL, Gunby J, et al. Increased risk of preterm birth in singleton pregnancies after blastocyst versus Day 3 embryo transfer: Canadian ART Register (CARTR) analysis. Hum Reprod 2013;28:924-8. CrossRef
19. Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stag embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2012;7:CD002118.
20. Debrock S, Spiessens C, Meuleman C, et al. New Belgian legislation regarding the limitation of transferable embryos in in vitro fertilization cycles does not significantly influence the pregnancy rate but reduces the multiple pregnancy rate in a threefold way in the Leuven University Fertility Center. Fertil Steril 2005;83:1572-4. CrossRef
21. Child TJ, Henderson AM, Tan SL. The desire for multiple pregnancy in male and female infertility patients. Hum Reprod 2004;19:558-61. CrossRef
22. Scotland GS, McNamee P, Peddie VL, Bhattacharya S. Safety versus success in elective single embryo transfer: women’s preferences for outcomes of in vitro fertilization. BJOG 2007;114:977-83. CrossRef
23. Hope N, Rombauts L. Can an educational DVD improve the acceptability of elective single embryo transfer? A randomized controlled study. Fertil Steril 2010;94:489-95. CrossRef