Hong Kong Med J 2014;20:70–3 | Number 1, February 2014
DOI: 10.12809/hkmj133879
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Scrotal wall metastasis as the first
manifestation of primary gastric adenocarcinoma
ST Leung, MB, BS, FRCR1; CY
Chu, FRCR, FHKAM (Radiology)1;
Billy MH Lai, MB, BS, FRCR1;
Florence MF Cheung, FRCPath, FHKAM (Pathology)2;
Jennifer LS Khoo, FRCR, FHKAM (Radiology)1
1 Department of Radiology,
Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Department of Clinical
Pathology, Pamela Youde Nethersole Eastern Hospital, Chai Wan,
Hong Kong
Corresponding author: Dr ST Leung (baryleung@hotmail.com)
Abstract
Metastases to the scrotal wall are very
rare, and being
the initial manifestation of occult primary tumours
is even rarer. We report on a patient presenting
with painless scrotal swelling, attributed to a solid
extra-testicular mass found on ultrasonography.
Subsequent investigations and surgical exploration
revealed it to be a scrotal wall metastasis from an
occult gastric primary. To our knowledge, this is
the first report of a scrotal wall metastasis from
gastric adenocarcinoma. The ensuing discussion and
literature review highlight the diagnostic challenges
posed by an extra-testicular scrotal metastasis from
an occult primary tumour.
Introduction
Metastases from gastric adenocarcinoma to
scrotal
structures are rare, most being intra-testicular. Extra-testicular
metastases are even rarer. It is extremely
rare for such a metastasis to be the first manifestation
of an occult primary tumour. Herein we report on a
patient who presented with a solid extra-testicular
mass, which was later confirmed to be a scrotal wall metastasis
from an occult gastric adenocarcinoma.
To our best knowledge, there has been no previous
report of a scrotal wall metastasis from gastric
adenocarcinoma.
Case report
A 66-year-old man, who enjoyed unremarkable
past
health, presented with a 2-week history of painless ‘right
testicular’ swelling in May 2011. Examination
yielded a 4-cm hard, irregular, and non-tender right
scrotal mass.
An urgent ultrasound revealed an 18 x 13 x
21-mm solid extra-testicular mass with heterogeneous
echogenicity in the right scrotum (Figs 1a-c), which
was separate from the normal-looking right testis.
Equivocal involvement of the right epididymis was
noted on the ultrasound at that time. Increased
vascularity of the mass lesion was noted on colour
Doppler study. A small right hydrocele was also
evident.
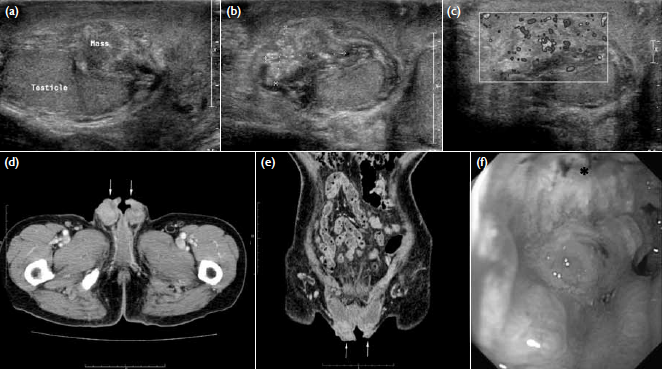
Figure 1. (a) Longitudinal and (b) transverse sonography of the right scrotum showing a solid extra-testicular mass separated from the normal-looking right testis. (c) Increased vascularity was evident on Doppler study. (d) Axial and (e) coronal computed tomographic images of the scrotum revealing bilateral scrotal soft-tissue thickening (arrows). (f) The oesophagogastroduodenoscopy reveals an irregular ulcerative tumour (*) over the lower part of gastric body
In view of a possible malignancy, the
patient
then underwent surgical scrotal exploration, which revealed a
markedly thickened scrotal wall. The dartos
muscles could not be well delineated and considered
a probable sight of invasion by tumour, though the
fat plane between the scrotal wall and the tunica was
preserved. The testes could be well separated from
the thickened scrotal wall.
A full-thickness scrotal wall incisional
biopsy
revealed diffuse infiltration of the subcutaneous
tissue and dartos muscle by aggregates and islands
of adenocarcinoma, more heavily on the right side.
Evidence of a desmoplastic reaction was noted. The
scrotal skin was unremarkable (Figs 2a-b).
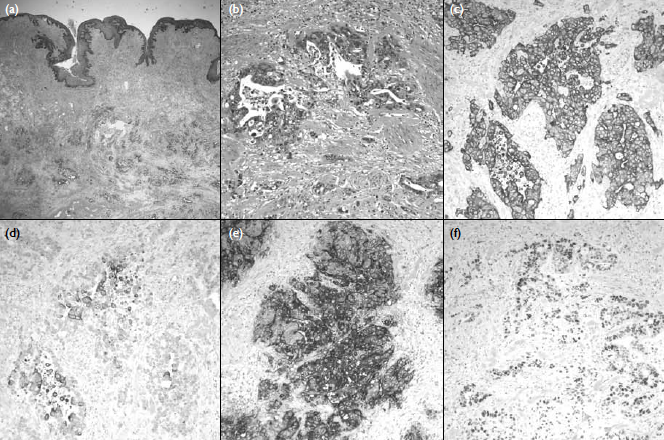
Figure 2. Pathological examination showing that (a) the soft tissue underlying the scrotal skin was infiltrated by adenocarcinoma (H&E, x 20) and (b) malignant glands lined by pleomorphic cells seen in the dartos muscle (H&E, x 200). Immunohistochemical studies (x 200) showing (c) tumour cells positive for CK7, but (d) only focally positive for CK20, and (e) strongly positive for carcinoembryonic antigen and (f) partly positive for p53. The overall features favour a tumour from the upper gastro-intestinal or genital origin
Immunological studies showed that the
tumour
cells were positive for CK7, carcinoembryonic
antigen, p53, and CDX2; focally positive for CK20
and negative for prostate-specific antigen (Figs 2c-f).
These features favoured a tumour of the upper gastro-intestinal
or genital origin. Biopsies of the urethra
and urinary bladder from the same operation were
negative of malignancy.
Subsequent computed tomography
(CT) of the abdomen and pelvis to search for
any underlying malignancy showed bilateral
scrotal soft tissue thickening (Figs 1d-e), and
oesophagogastroduodenoscopy revealed a hard,
irregular, and circumferential ulcerative tumour over
the lower part of body of stomach (Fig 1f). Biopsy of this ulcerative tumour
revealed a poorly differentiated
adenocarcinoma.
Palliative chemotherapy with the XELOX
regimen (capecitabine plus oxaliplatin) was started.
The chemotherapy regimen was subsequently changed
to the FOLFOX regimen (folinic acid, fluorouracil,
and oxaliplatin) because of progression of the gastric
malignancy. He remained otherwise asymptomatic
10 months following the initial presentation, when
this report was submitted for publication.
Discussion
The scrotum is a musculocutaneous sac
composed
of two compartments, divided by a midline septum.
Each compartment contains a testis, epididymis,
spermatic cord, and associated fascial coverings.1
The scrotal wall is composed of pigmented skin,
subcutaneous tissue, and the closely related dartos
fascia and dartos muscle.
Metastases to scrotal structures are rare.
Among these, the testis is the most frequently
involved site. Less than 3% of testicular malignancies
are secondaries; the lung, prostate, and gastro-intestinal
tract are the most common primary sites.2
3
Metastases to extra-testicular scrotal structures
such as the epididymis, spermatic cord, and scrotal
wall are even rarer. Metastases account for 8.1% of
malignancies in the epididymis and spermatic cord,3 4
mostly reported as single case reports.3
Metastasis to the scrotal wall involving
the
subcutaneous tissue and dartos muscle with normal
scrotal skin (as in our patient) is extremely rare. A
previous review could only identify sporadic cases
of scrotal wall metastases; the primaries being from
malignant melanoma, anal carcinoma, and lung
carcinoma.5 To our best
knowledge, no scrotal wall
metastasis from gastric adenocarcinoma has ever
been reported in the literature. On the other hand,
cutaneous metastases involving the scrotal skin alone
are relatively more common. In contrast to scrotal
wall metastases which present as a scrotal swelling,
scrotal skin metastases present as cutaneous polypoid
lesions, ulcers, or papules.6
7 8
Several pathways by which primary tumours
metastasise to the scrotal structures have been
proposed. They include retrograde venous
embolism, retrograde lymphatic extension, arterial
embolism, direct invasion along the testicular cord,
and transperitoneal seeding through a congenital
hydrocele.9 Although the
exact pathway of the
metastasis in our patient is not clear, absence of
intra-abdominal, pelvic lymphadenopathy, and
testicular cord thickening all favour embolism or
transperitoneal seeding as the route.
Clinically, a scrotal wall metastasis
usually
presents as a painless scrotal swelling with normal
overlying skin, with a firm-to-hard mass being evident
on physical examination. Ultrasonography usually reveals a solid
extra-testicular mass, that is mostly
hypoechoic but can have variable echogenicity.2
Previously described sonographic features of a scrotal
wall metastasis from a lung primary also entailed
increased peripheral vascularity and poor delineation
with the epididymis,2 in
which the features were also
observed in our patient.
Ultrasonography is usually the first
imaging
modality for evaluating patients presenting with
scrotal swelling. Its high spatial resolution provides
nearly 100% sensitivity in identifying a scrotal mass
and a 98 to 100% sensitivity in differentiating intra-testicular
versus extra-testicular lesions.1
The two
most important factors to consider during evaluation
of a scrotal mass are whether it is intra- or extra-testicular
in location, and whether it is cystic or solid
in nature.1 This is because
more than 95% intra-testicular
masses are malignant while most that are
extra-testicular are benign.10
Most extra-testicular masses are cystic and
almost all are benign.1
Solid extra-testicular masses
are uncommon and 97% of them are also benign.1 11
Among these solid extra-testicular masses, lipoma
and adenomatoid tumours are most frequently
encountered, and account for about 45% and 33% of
all extra-testicular masses, respectively.11
Malignancies account for the remaining 3%
of
extra-testicular masses. It was estimated that 8.1%
of malignancies in the epididymis and spermatic
cord are due to metastases.3
4 Rhabdomyosarcoma
is the most common primary malignant tumour,
accounting for 40% of all malignant extra-testicular
tumours and are most common in infants and
children.11 In adults,
examples of primary malignant
tumours include leiomyosarcoma, liposarcoma,
fibrosarcoma, malignant fibrous histiocytoma, and
primary adenocarcinoma of epididymis.5
Secondary extra-testicular malignant
tumours
usually occur against a background of known
advanced malignancy. Common primary sites
include prostate, kidney, gastro-intestinal tract,
and pancreas.11 Epididymis
and spermatic cord
are the usual sites of involvement, while scrotal
wall metastases are extremely rare. Only sporadic
case reports can be identified in the literature for
metastases to the scrotal wall (from melanoma, anal
carcinoma, and lung carcinoma).2
12
Lesion diameter, volume, and presence of
vascularity on Doppler ultrasonography assist
differentiation of malignant from benign lesions.13
Multifocal lesions and heterogeneity have also been
suggested as supporting metastatic disease.14 15
However, in most instances, considerable overlap
in sonographic appearances of many solid extra-testicular
masses preclude a specific diagnosis.1
Magnetic resonance imaging (MRI) is useful
in
characterising certain extra-testicular lesions such
as lipoma, haematoma, and fibrous pseudotumour. Enhancement
patterns in gadolinium-enhanced
MRIs are also useful in differentiating malignant
from benign lesions. In such cases, MRI findings may
obviate the need for surgery or change the surgical
approach.14
In patients without a known history of
malignancy such as ours, diagnosis of an extra-testicular
metastasis on the initial ultrasound is
difficult. The sonographic differential diagnosis
includes other extra-testicular benign and malignant
tumours. Clinical correlation is essential to enable
better differentiation of malignant from benign
lesions. The clinical finding of a hard scrotal mass
in our patient raised concerns of malignancy, and
hence surgical exploration was undertaken. The final
diagnosis still depends on biopsy and pathological
study. In our patient, histology and immunological
studies of the scrotal wall biopsy hinted at the
final diagnosis of occult gastric adenocarcinoma.
Although positron emission tomography–CT may
be useful for seeking an occult primary malignancy,
it is not commonly used as a first-line imaging
modality in patients presenting with a scrotal
wall lesion. However, it could be offered to search
for an underlying primary when histology shows
adenocarcinoma but immunological study results are
pending.
Conclusion
Extra-testicular metastases are rare and
have non-specific
sonographic features, which always pose
difficulties in diagnosis, particularly in patients
without a known primary malignancy. We hereby
report the first case of a gastric adenocarcinoma
presenting as scrotal wall metastasis. This case also
demonstrates the importance of radiological, clinical,
and pathological correlations in making the final
diagnosis.
References
1. Woodward PJ, Schwab CM,
Sesterhenn IA. From the archives of the AFIP: extratesticular
scrotal masses: radiologic-pathologic correlation. Radiographics
2003;23:215-40. Crossref
2. Dogra V, Saad W, Rubens DJ.
Sonographic appearance of scrotal wall metastases from lung
adenocarcinoma. AJR Am J Roentgenol 2002;179:1647-8. Crossref
3. Dutt N, Bates AW, Baithun SI.
Secondary neoplasms of the male genital tract with different
patterns of involvement in adults and children. Histopathology
2000;37:323-31. Crossref
4. Algaba F, Santaularia JM,
Villavicencio H. Metastatic tumor of the epididymis and spermatic
cord. Eur Urol 1983;9:56-9.
5. Dogra VS, Gottlieb GH, Oka M,
Rubens DJ. Sonography of the scrotum. Radiology 2003;227:18-36. Crossref
6. Aridogan IA, Satar N, Doran E,
Tansug MZ. Scrotal skin metastases of renal cell carcinoma: a case
report. Acta Chir Belg 2004;104:599-600.
7. McWeeney DM, Martin ST, Ryan RS,
Tobbia IN, Donnellan PP, Barry KM. Scrotal metastases from
colorectal carcinoma: a case report. Cases J 2009;2:111. Crossref
8. Wang SQ, Mecca PS, Myskowski PL,
Slovin SF. Scrotal and penile papules and plaques as the initial
manifestation of a cutaneous metastasis of adenocarcinoma of the
prostate: case report and review of the literature. J Cutan Pathol
2008;35:681-4. Crossref
9. Hanash KA, Carney JA, Kelalis
PP. Metastatic tumors to the testicles: routes of metastasis. J
Urol 1969;102:465-8.
10. Moghe PK, Brady AP. Ultrasound
of testicular epidermoid cysts. Br J Radiol 1999;72:942-5.
11. Akbar SA, Sayyed TA, Jafri SZ,
Hasteh F, Neill JS. Multimodality imaging of paratesticular
neoplasms and their rare mimics. Radiographics 2003;23:1461-76. Crossref
12. Ferguson MA, White BA, Johnson
DE, Carrington PR, Schaefer RF. Carcinoma en cuirasse of the
scrotum: an unusual presentation of lung carcinoma metastatic to
the scrotum. J Urol 1998;160(6 Pt 1):2154-5.
13. Alleman WG, Gorman B, King BF,
Larson DR, Cheville JC, Nehra A. Benign and malignant epididymal
masses evaluated with scrotal sonography: clinical and pathologic
review of 85 patients. J Ultrasound Med 2008;27:1195-202.
14. Cassidy FH, Ishioka KM,
McMahon CJ, et al. MR imaging of scrotal tumors and pseudotumors.
Radiographics 2010;30:665-83. Crossref
15. Souza FF, Di Salvo D.
Sonographic features of a metastatic extratesticular
gastrointestinal stromal tumor. J Ultrasound Med 2008;27:1639-42.

