Hong Kong Med J 2025;31:Epub 12 Nov 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Use of 18F-fluorodeoxyglucose positron emission
tomography coupled with computed tomography
in early breast cancer management: consensus-based
local recommendations by the Hong Kong
Breast Cancer Foundation PET/CT Study Group
Carol CH Kwok, MB, ChB, FHKAM (Radiology)# † 1; Henry CY Wong, MB, BS, FHKAM (Radiology)# † 1; Catherine YH Wong, MB, BS, FHKAM (Radiology)† 2; LW Yuen, MS, MA3; CC Yau, MB, BS, FHKAM (Radiology)† 3; Polly SY Cheung, MB, BS, FHKAM (Surgery)† 3
1 Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
2 Department of Nuclear Medicine, Hong Kong Sanatorium & Hospital, Hong Kong SAR, China
3 Hong Kong Breast Cancer Foundation, Hong Kong SAR, China
# Equal contribution
† Members of the Hong Kong Breast Cancer Foundation PET/CT Study Group
Corresponding author: Dr Carol CH Kwok (kwokch@ha.org.hk)
Abstract
Introduction: 18F-fluorodeoxyglucose positron
emission tomography coupled with computed
tomography (PET/CT) has been incorporated into
breast cancer management. In Hong Kong, PET/CT
use is increasing. This study aimed to establish
consensus-based recommendations on the use of
PET/CT in the management of early breast cancer.
Methods: A literature search was conducted in
September 2023 using the keywords “breast cancer”
and “PET/CT” within PubMed to identify research
articles related to the use of PET/CT in early breast
cancer. Guidelines from major international cancer
agencies were also reviewed. Ten recommendation
statements were drafted. A two-round modified
Delphi consensus process was conducted over a
3-month period (19 December 2023 to 29 February
2024).
Results: A total of 76 experts consented to participate
in the first round, of whom 71 completed the second
round and were included as members of the expert
panel, yielding a second-round response rate of
93.4%. The panel comprised oncologists (n=30,
42.3%), surgeons (n=35, 49.3%), and radiologists
(including nuclear medicine radiologists) [n=6,
8.5%]. Experts from the Hospital Authority (n=37,
52.1%) and the private sector (n=32, 45.1%) were
well represented. Two experts (2.8%) were from one
of the two local university medical faculties. Over 75% of expert panel members had at least 15 years of
clinical experience. Of the ten statements, consensus
was achieved on seven in the first round and one
additional statement in the second round.
Conclusion: Through the consensus process, the
proposed recommendations are expected to gain
wider acceptance and recognition among local
healthcare professionals as guidance for the use of
PET/CT in early breast cancer management.
New knowledge added by this study
- First-of-its-kind local consensus-based recommendations on the use of positron emission tomography coupled with computed tomography (PET/CT) in early breast cancer were established.
- The proposed recommendations were based on the largest and most up-to-date evidence, which reflected updated international guideline recommendations.
- The consensus-establishing process provided a platform for exchange and sharing among multidisciplinary teams in resolving controversial aspects of clinical practice.
- Local recommendations on the use of PET/CT for early breast cancer patients have been proposed in light of the increasing availability of PET/CT facilities in Hong Kong.
- These consensus recommendations cover important and relevant clinical settings, including screening, preoperative assessment of multifocality, axillary staging, pretreatment staging, evaluation of tumour response and axillary nodal status in the neoadjuvant setting before surgery, re-staging in recurrence, and follow-up for surveillance.
- Through the consensus process, the proposed recommendations are expected to gain wider acceptance and recognition among local healthcare professionals as guidance on the use of PET/CT in early breast cancer management.
Introduction
Diagnostic imaging plays an important role in the
screening, diagnosis, staging, and follow-up of
patients affected by breast cancer. Mammography
and breast ultrasound are the current standards
of care for screening, diagnosis, and surveillance.
For patients with locally advanced disease,
guidelines recommend contrast-enhanced
computed tomography (CT) scans and bone scans
to detect distant metastases. In recent years, 18F-fluorodeoxyglucose
(18F-FDG) positron emission
tomography coupled with CT (PET/CT) has been
introduced as an important imaging modality in
oncological care. It is a powerful tool that combines
the spatial resolution of a CT scan with information
regarding biological processes within the scanned
region. Positron emission tomography coupled
with CT has the potential to identify malignant
disease that may otherwise be missed or classified
as benign based on size or morphological features in
conventional imaging modalities.
In 2021, the Hong Kong Breast Cancer
Foundation (HKBCF) analysed the utilisation of
PET/CT among patients enrolled in the Hong Kong
Breast Cancer Registry since 2007. Among the 4154
patients studied, the utilisation rate of PET/CT was 40.4% (online supplementary Fig 1). There was
an increasing trend in PET/CT scan use for breast
cancer staging over the past two decades. The overall
utilisation of PET/CT increased from 23.3% in 2006-2010, to 48.5% in 2011-2015, and to 61.6% in the
2016-2021 cohort across all cancer stages (online supplementary Fig 2). This trend largely reflected the
increasing availability of PET/CT facilities in Hong
Kong. Over the past two decades, multiple PET/CT
scanning facilities have been established in both the
public and private sectors, making the service more
accessible. Overall, usage of PET/CT was correlated
with higher pathological stages of disease. Notably,
PET/CT was used in up to 13.8% of stage 0 cases and
21.0% of stage I cases (online supplementary Fig 3).
Given the relatively high costs, concerns
regarding radiation exposure, and the possibility
of false-negative results, it is important to provide
local recommendations on which groups of patients
would benefit from the use of PET/CT in breast
cancer. Through this study, we aimed to develop a
local guideline regarding the use of PET/CT for early
breast cancer to assist healthcare professionals in
making evidence-based recommendations.
Methods
The objective of this study was to develop local
recommendations on how to utilise PET/CT in the
screening, diagnosis, staging, treatment response
assessment, and surveillance of early breast cancer.
A study group consisting of five members from the
HKBCF (first, second, third, fifth and sixth authors)
was convened. Study Group members were involved
in performing the literature search, constructing the
Delphi survey, analysing data, interpreting findings,
and providing final approval of the recommendations.
To construct the survey, a literature search
was performed in September 2023 by the Study
Group using the keywords “breast cancer” and
“PET/CT” in PubMed to identify research articles
related to the use of PET/CT in early breast cancer.
Systematic reviews and randomised controlled
trials were prioritised to form the evidence base
for the proposed statements. Guidelines from
major international cancer agencies, including the
National Comprehensive Cancer Network (NCCN)
and the European Society for Medical Oncology,
were reviewed. Ten statements were drafted based
on the literature and international guidelines.
Delphi consensus process
A two-round modified Delphi consensus process was
conducted over a period of 3 months (19 December
2023 to 29 February 2024). Surveys were developed
using Google Forms, a web-based development tool.
Responses provided by individual participants were
anonymised to protect confidentiality. This study did not involve any patients as participants. Only
individuals who took part in the first round were
invited to participate in the second round.
Experienced physicians with an interest in
breast cancer, working in the medical faculties of
The University of Hong Kong and The Chinese
University of Hong Kong, the Hospital Authority,
and the private sector, were identified by the Study
Group and invited to participate in the Delphi
process. Additionally, members of the Hong Kong
Breast Cancer Registry Steering Committee, the
Hong Kong Breast Oncology Group, and the Hong
Kong Society of Breast Surgeons were invited. Emails
were sent to all potential participants by the Study
Group to confirm their interest in participating.
After providing informed consent, participants
were directed to an online survey for completion.
In the first round, participants were provided with
a summary of evidence corresponding to each of
the ten statements in the survey (online Appendix 1). Participants were asked to indicate the extent
of their agreement or disagreement on a five-point
Likert scale (‘Completely agree’, ‘Agree’, ‘Neutral’,
‘Disagree’, and ‘Completely disagree’) for each
statement. Respondents who selected ‘Disagree’ or
‘Completely disagree’ were asked to provide reasons
for their choice in a free-text field within the survey.
In accordance with published recommendations,
statements that achieved agreement (‘Completely
agree’ or ‘Agree’) from more than 75% of participants
were considered to have reached consensus.
Following participant voting, the Study Group
compiled and prepared the results from the first
round. Statements that did not reach consensus
were reviewed and amended based on participant
feedback. For the second round, statements that
did not reach consensus, or were newly created or
modified based on participant feedback, were sent as
a survey to the same participants. Participants were
shown the results of the first round and informed
where amendments had been made to statements in
the second round.
Consensus statement disclaimer
The recommendations provided in this publication
reflect the majority opinion of the expert panel.
Although the recommendations are intended to
guide clinical decision-making, they should not
be regarded as the sole indications for utilising
PET/CT in early breast cancer management. These
consensus-based recommendations are designed to
provide guidance for oncologists, surgeons, general
practitioners, radiologists, and other physicians
involved in the care of patients with early breast
cancer. Treatment decisions for individual patients
should ultimately be made at the discretion of the
treating clinician, in conjunction with the patient’s
unique needs and through shared decision-making.
Results
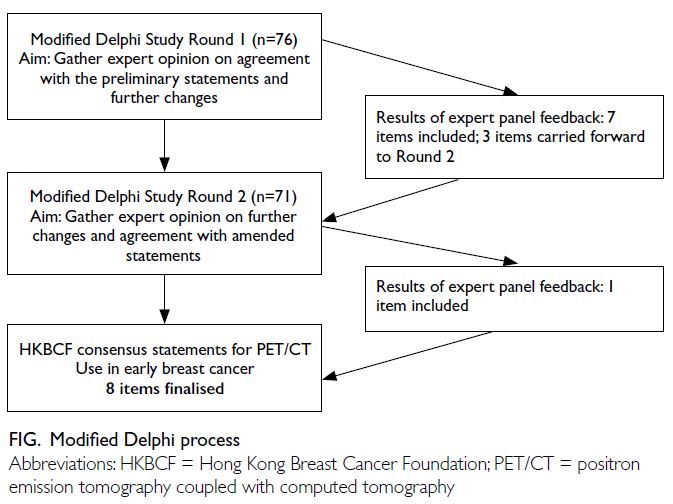
Two Delphi consensus rounds were completed.
Among the 270 invited experts, 76 consented to
participate in the first round, of whom 71 completed
the second round and were included as members of
the expert panel (online Appendix 2). The response
rate for the second round was 93.4%. The panel
comprised oncologists (n=30, 42.3%), surgeons
(n=35, 49.3%), and radiologists (including nuclear
medicine radiologists) [n=6, 8.5%]. Experts from the
Hospital Authority (n=37, 52.1%) and the private
sector (n=32, 45.1%) were well represented. Two
experts (2.8%) were from one of the two medical
faculties of the local universities. Over 75% of expert
panel members had at least 15 years of clinical
experience.
Of the ten statements, consensus was achieved
on seven in the first round. Three statements were
returned to the expert panel for rating in the second
round, of which one achieved consensus (Fig). The
results of the final consensus on the recommendation
statements after the two-round Delphi consensus
process are listed in the Table.
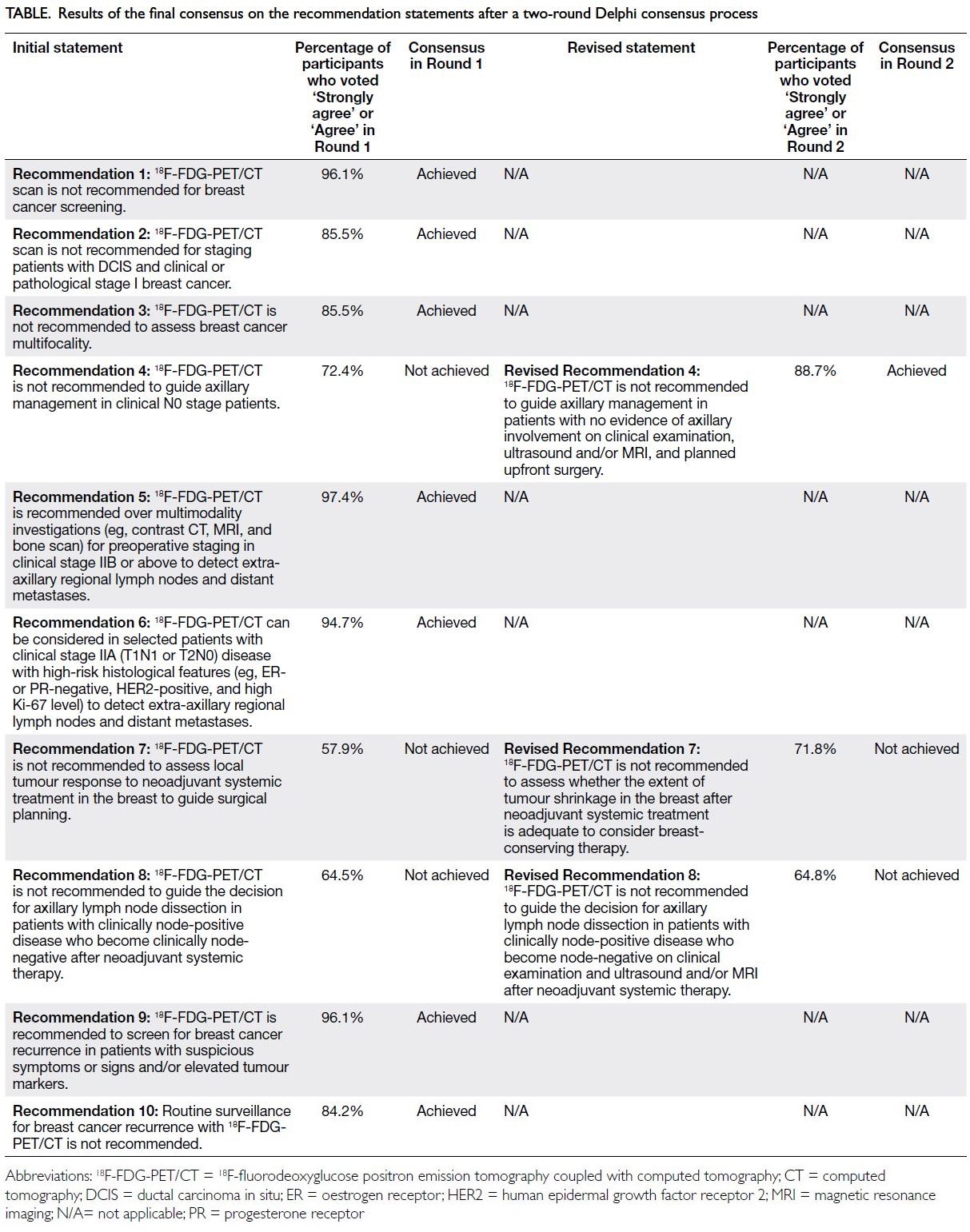
Table. Results of the final consensus on the recommendation statements after a two-round Delphi consensus process
Discussion
In recent years, driven by increasing demand
and easier access to PET/CT services, there has
been a substantial increase in the use of PET/CT
for breast cancer patients. Currently, there are
33 PET/CT machines across public, private, and
academic institutions in Hong Kong. While PET/CT
has the capability to enhance the detection of
occult malignant disease, it also carries the risk of
identifying false-positives and incidental findings, which could lead to unnecessary investigations
and potentially delay curative-intent treatments.
Although the utility of PET/CT in various breast
cancer settings has been widely studied, there
remains a lack of large prospective randomised
studies comparing it with other imaging modalities.
Given that PET/CT is costly and poses concerns
about increased radiation exposure compared with
other imaging techniques, such as contrast-enhanced
CT scans, the development of local guidance and
recommendations regarding its indications is
clinically relevant and essential. To our knowledge,
this consensus-based guideline is the first to provide
practical recommendations on the use of PET/CT
for breast cancer management.
Of the ten recommendation statements
proposed, seven achieved consensus in the first
round, suggesting that the indications for PET/CT in
these areas are clear-cut and less controversial. These
statements covered areas related to the screening,
diagnosis, staging, and surveillance of breast cancer.
Overall, the majority of local experts agreed that
PET/CT should only be utilised in situations where
patients have a high risk of distant metastases. This
approach includes staging patients with advanced
clinical stage disease or aggressive tumour biology
and evaluating cancer survivors with suspicious
clinical signs and symptoms suggestive of recurrence.
Conversely, PET/CT should not be used in situations
where the likelihood of detecting malignant disease
is low, such as staging of ductal carcinoma in
situ or stage I disease, screening asymptomatic
women for breast cancer, and routine surveillance
of cancer survivors. Increased 18F-FDG avidity of
malignant cells forms the basis of 18F-FDG-PET
in breast cancer imaging. Tumour characteristics
that limit the sensitivity of 18F-FDG-PET in breast
cancer imaging include small tumour size, low
tumour grade, low proliferation, high expression
of hormone receptors (particularly luminal A
phenotype), and lobular histological type.1 2 3 Positron
emission tomography coupled with CT therefore
has limited sensitivity in detecting subcentimetre
tumours,4 5 micrometastases, and small lymph node
metastases in a clinically negative axilla relative to
sentinel lymph node biopsy (SLNB).6 7 Additionally,
the specificity of PET/CT is affected—some benign
tumours and infectious or inflammatory conditions
can demonstrate 18F-FDG uptake.8 Positron emission
tomography coupled with CT has limited spatial
resolution in assessing the multifocality of breast
cancer.9
In contrast to its low sensitivity for detecting
axillary nodal metastases, 18F-FDG PET/CT
demonstrates high sensitivity in detecting extra-axillary
lymph node involvement, including internal
mammary, infraclavicular, and supraclavicular
nodes10 11; distant metastases; and other unsuspected synchronous malignancies during initial breast
cancer staging, which can potentially lead to
upstaging and ultimately modification of planned
treatment.12 13 14 The detection of extra-axillary lymph
node involvement aids in selecting candidates for
neoadjuvant chemotherapy and may guide subsequent
radiotherapy planning to ensure adequate coverage
of nodal involvement sites.11 15 16 In contrast to stage
0 and stage I disease, where the likelihood of distant
metastasis is low, there is a growing body of evidence
that PET/CT may outperform conventional imaging
(contrast-enhanced CT of the thorax, abdomen, and
pelvis; and bone scan).17 18 Furthermore, high-grade
and poor-risk cancer subtypes may exhibit increased
18F-FDG uptake, thereby enhancing the diagnostic
yield of PET/CT in staging these tumours.19 20 21 Our recommendations align with those of the NCCN22
and the French working group,23 which recently updated their guidance in this regard.
Controversies
The two recommendation statements that did not
reach consensus after the Delphi rounds related to
post–neoadjuvant therapy evaluation of tumour
response to guide surgery to the primary tumour and
axilla. In recent years, neoadjuvant chemotherapy
has been increasingly used to downstage disease,
facilitate surgery, and provide an opportunity
for in vivo tumour response assessment to guide
individualised treatment escalation or de-escalation
after surgery. This approach has become the standard
of care for patients with larger tumours who wish
to undergo breast-conserving therapy and for stage
II and III patients with aggressive tumour biology
(eg, triple-negative and human epidermal growth
factor receptor 2–positive breast cancer).22 Current
studies on post-neoadjuvant chemotherapy tumour
response assessment have mainly focused on the
prediction of pathological complete response.24 25 26 27
Previous studies have shown that magnetic
resonance imaging (MRI) may exhibit higher
sensitivity, whereas PET/CT demonstrates higher
specificity in predicting the pathological response
after neoadjuvant chemotherapy, indicating the
complementary value of combining these modalities
to improve diagnostic performance.28
The method of assessing primary tumour
response during neoadjuvant therapy has varied
across clinical trials. For example, in the NeoSphere
trial, which evaluated the addition of neoadjuvant
pertuzumab to docetaxel and trastuzumab, clinical
response was assessed via physical examination.29
Other trials have supplemented clinical assessment
with diagnostic imaging during treatment. In the
PREDIX HER2 trial, which compared neoadjuvant
docetaxel, trastuzumab and pertuzumab versus
trastuzumab emtansine, investigators routinely
utilised mammography, ultrasound, or MRI after the second, fourth, and sixth cycles for response
assessment.30 Positron emission tomography coupled
with CT was performed at baseline, then repeated
after the second and final cycles at the investigators’
discretion.30 Currently, international guidelines vary
in their recommendations of preferred assessment
modality. The 2024 European Society for Medical
Oncology guideline31 recommends the use of MRI
to assess local response if pretreatment MRI data
are available. The NCCN guidelines22 suggest that
assessment should include physical examination
and imaging studies, with the choice of imaging
modality determined by a multidisciplinary team.
The differing opinions within our expert panel reflect
these variations in existing evidence and guidelines.
Clinicians should individualise their assessment
strategy based on the patient’s clinical status and
access to imaging modalities.
It has long been the standard of care to
offer axillary lymph node dissection to patients
with a clinically positive axillary lymph node to
ensure adequate tumour clearance. However,
given the introduction of neoadjuvant systemic
therapies, ongoing studies are evaluating alternative
approaches to axillary management to reduce the
risk of arm lymphoedema. In patients who have
converted from clinically node-positive to clinically
node-negative disease after systemic therapy,
SLNB and targeted axillary lymph node dissection
are currently recommended by international
guidelines (instead of routine axillary lymph node
dissection).22 Our Delphi study surveyed the views
of local experts on whether PET/CT should be
recommended as an additional imaging modality
to screen for occult residual axillary disease. While
recognising that PET/CT may yield false-positive
results, some experts reported using PET/CT to
guide whether axillary lymph node dissection could
be undertaken directly without a positive SLNB,
particularly in patients with initially bulky axillary
disease. This approach aligns with the latest NCCN
guidelines,22 which caution against the use of SLNB in pre-chemotherapy clinical N2 stage disease. The
statement that PET/CT is not recommended to
guide the decision for axillary lymph node dissection
in patients with clinically node-positive disease who
become node-negative on clinical examination and
ultrasound and/or MRI after neoadjuvant systemic
therapy remains open. Further studies regarding
the accuracy of PET/CT in this context may help
resolve the controversy. The management approach
for the axilla after neoadjuvant therapy is constantly
evolving. For example, axillary radiation is currently
being tested as an alternative to axillary lymph
node dissection in the ongoing Alliance A011202
randomised trial among patients with a positive
SLNB.32 The timing and role of PET/CT will need to be re-evaluated within this ever-changing paradigm of axillary management in the neoadjuvant setting.
Positron emission tomography coupled with
CT is often presumed to involve high radiation
exposure. However, when used appropriately for
breast cancer staging with low-dose, non-contrast
CT, the radiation exposure can be considerably
lower than that of whole-body, high-resolution
contrast CT combined with a bone scan. Previous
international guidelines have suggested that PET/CT
can be performed in situations where standard
staging studies are equivocal or suspicious.22 31 Such
a sequential approach may not be cost-effective in
the clinical scenarios outlined by our expert panel
and may expose patients to unnecessary radiation
from multiple whole-body imaging examinations.
The use of PET/CT as a one-stop assessment enables
quicker evaluation of disease status and can facilitate
earlier initiation of appropriate treatment.33
Strengths and limitations
A strength of our Delphi consensus study is that it
involved a large group of experienced specialists
representing multiple disciplines and both the
public and private sectors. This consensus exercise
provided a valuable platform in which clinical
experiences, practices, ideas, and opinions were
shared and exchanged anonymously. It also helped
resolve controversial issues and achieve consensus,
particularly in areas where high-level evidence
is absent. Recommendations that have achieved
consensus should receive wider acceptance and
recognition when incorporated into clinical practice.
However, our study had notable limitations.
First, expert panellists were invited by the Study
Group, and thus the consensus results may not fully
reflect the views of all local practitioners involved
in treating breast cancer patients. Nevertheless,
our sample size of more than 70 participants is
considered large for Delphi studies, and we achieved
balanced representation of participants from various
backgrounds. Second, the initial statements were
devised based on recently published articles selected
by the Study Group, which could introduce bias
compared with a formal systematic review. However,
the Study Group prioritised reviewing meta-analyses
and randomised controlled trials when drafting the
initial statements to ensure they reflected the most
up-to-date, high-level evidence.
Conclusion
Based on the results of this Delphi consensus
study, the HKBCF PET/CT Study Group provides
recommendations on the use of PET/CT for early
breast cancer in areas of screening, diagnosis,
staging, and surveillance. These recommendations
are intended to guide the appropriate use of PET/CT
in the local population across both public and private healthcare settings. Breast cancer management is
rapidly advancing, and the management paradigm
is continually evolving as new evidence becomes
available. As technology progresses, more innovative
imaging modalities, such as PET/MRI and PET
scans with new radiotracers, are expected to play an
increasing role.14 34 35 The Study Group will review and
update these recommendation guidelines at regular
intervals based on emerging evidence, particularly
in relation to response assessment during and after
neoadjuvant systemic therapy.
Author contributions
Concept or design: PSY Cheung, CC Yau, CCH Kwok, HCY Wong, CYH Wong.
Acquisition of data: CCH Kwok, HCY Wong.
Analysis or interpretation of data: HCY Wong, CCH Kwok, LW Yuen.
Drafting of the manuscript: CCH Kwok, HCY Wong.
Critical revision of the manuscript for important intellectual content: CCH Kwok, HCY Wong, CYH Wong, CC Yau, PSY Cheung.
Acquisition of data: CCH Kwok, HCY Wong.
Analysis or interpretation of data: HCY Wong, CCH Kwok, LW Yuen.
Drafting of the manuscript: CCH Kwok, HCY Wong.
Critical revision of the manuscript for important intellectual content: CCH Kwok, HCY Wong, CYH Wong, CC Yau, PSY Cheung.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank all participants who contributed to this research.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Breast Cancer Research
Centre Research Committee of the Hong Kong Breast
Cancer Foundation. The requirement for informed consent
from patients was waived by the Committee as patient data
collection by the Hong Kong Breast Cancer Registry was
approved by respective participating hospitals and centres.
The present study does not involve patient participation and
there was no new patient data collection.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Groheux D, Giacchetti S, Moretti JL, et al. Correlation of
high 18F-FDG uptake to clinical, pathological and biological
prognostic factors in breast cancer. Eur J Nucl Med Mol
Imaging 2011;38:426-35. Crossref
2. Buck A, Schirrmeister H, Kühn T, et al. FDG uptake in
breast cancer: correlation with biological and clinical
prognostic parameters. Eur J Nucl Med Mol Imaging
2002;29:1317-23. Crossref
3. Humbert O, Berriolo-Riedinger A, Cochet A, et al.
Prognostic relevance at 5 years of the early monitoring of
neoadjuvant chemotherapy using 18F-FDG PET in luminal
HER2-negative breast cancer. Eur J Nucl Med Mol Imaging
2014;41:416-27. Crossref
4. Avril N, Rosé CA, Schelling M, et al. Breast imaging
with positron emission tomography and fluorine-18
fluorodeoxyglucose: use and limitations. J Clin Oncol
2000;18:3495-502. Crossref
5. Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M,
Alavi A. Clinicopathologic factors associated with false
negative FDG-PET in primary breast cancer. Breast Cancer
Res Treat 2006;98:267-74. Crossref
6. Peare R, Staff RT, Heys SD. The use of FDG-PET in assessing
axillary lymph node status in breast cancer: a systematic
review and meta-analysis of the literature. Breast Cancer
Res Treat 2010;123:281-90. Crossref
7. Cooper KL, Harnan S, Meng Y, et al. Positron emission
tomography (PET) for assessment of axillary lymph node
status in early breast cancer: a systematic review and meta-analysis.
Eur J Surg Oncol 2011;37:187-98. Crossref
8. Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive
lesions mimicking breast cancer on FDG PET and
PET/CT. AJR Am J Roentgenol 2012;198:W304-14. Crossref
9. Ergul N, Kadioglu H, Yildiz S, et al. Assessment of
multifocality and axillary nodal involvement in early-stage
breast cancer patients using 18F-FDG PET/CT compared
to contrast-enhanced and diffusion-weighted magnetic
resonance imaging and sentinel node biopsy. Acta Radiol
2015;56:917-23. Crossref
10. Aukema TS, Straver ME, Peeters MJ, et al. Detection of
extra-axillary lymph node involvement with FDG PET/CT
in patients with stage II–III breast cancer. Eur J Cancer
2010;46:3205-10. Crossref
11. Seo MJ, Lee JJ, Kim HO, et al. Detection of internal mammary
lymph node metastasis with 18F-fluorodeoxyglucose
positron emission tomography/computed tomography in
patients with stage III breast cancer. Eur J Nucl Med Mol
Imaging 2014;41:438-45. Crossref
12. Rong J, Wang S, Ding Q, Yun M, Zheng Z, Ye S. Comparison
of 18FDG PET-CT and bone scintigraphy for detection of
bone metastases in breast cancer patients. A meta-analysis.
Surg Oncol 2013;22:86-91. Crossref
13. Sun Z, Yi YL, Liu Y, Xiong JP, He CZ. Comparison of
whole-body PET/PET-CT and conventional imaging
procedures for distant metastasis staging in patients with
breast cancer: a meta-analysis. Eur J Gynaecol Oncol
2015;36:672-6.
14. Han S, Choi JY. Impact of 18F-FDG PET, PET/CT, and
PET/MRI on staging and management as an initial staging
modality in breast cancer: a systematic review and metaanalysis.
Clin Nucl Med 2021;46:271-82. Crossref
15. Groheux D, Espié M, Giacchetti S, Hindié E. Performance
of FDG PET/CT in the clinical management of breast cancer. Radiology 2013;266:388-405. Crossref
16. Borm KJ, Voppichler J, Düsberg M, et al. FDG/PET-CT–based lymph node atlas in breast cancer patients. Int J
Radiat Oncol Biol Phys 2019;103:574-82. Crossref
17. Caresia Aroztegui AP, García Vicente AM, Alvarez Ruiz S,
et al. 18F-FDG PET/CT in breast cancer: evidence-based
recommendations in initial staging. Tumor Biol
2017;39:1010428317728285. Crossref
18. Dayes IS, Metser U, Hodgson N, et al. Impact of 18F-labeled
fluorodeoxyglucose positron emission tomography–computed tomography versus conventional staging in
patients with locally advanced breast cancer. J Clin Oncol 2023;41:3909-16. Crossref
19. de Mooij CM, Ploumen RA, Nelemans PJ, Mottaghy FM,
Smidt ML, van Nijnatten TJ. The influence of receptor
expression and clinical subtypes on baseline [18F]FDG
uptake in breast cancer: systematic review and meta-analysis.
EJNMMI Res 2023;13:5. Crossref
20. Basu S, Chen W, Tchou J, et al. Comparison of triple-negative
and estrogen receptor–positive/progesterone
receptor–positive/HER2-negative breast carcinoma using
quantitative fluorine-18 fluorodeoxyglucose/positron
emission tomography imaging parameters: a potentially
useful method for disease characterization. Cancer
2008;112:995-1000. Crossref
21. Ulaner GA, Castillo R, Goldman DA, et al. 18F-FDG-PET/CT for systemic staging of newly diagnosed triple-negative breast cancer. Eur J Nucl Med Mol Imaging 2016;43:1937-44. Crossref
22. Gradishar WJ, Moran MS, Abraham J, et al. NCCN
Guidelines® Breast Cancer Version 4.2023. J Natl Compr
Canc Netw 2023;21:594-608. Crossref
23. Groheux D, Hindie E. Breast cancer: initial workup
and staging with FDG PET/CT. Clin Transl Imaging
2021;9:221-31. Crossref
24. Elsayed B, Alksas A, Shehata M, et al. Exploring
neoadjuvant chemotherapy, predictive models, radiomic,
and pathological markers in breast cancer: a comprehensive
review. Cancers 2023;15:5288. Crossref
25. Imbriaco M, Ponsiglione A. Predicting pathologic complete
response after neoadjuvant chemotherapy. Radiology
2021;299:301-2. Crossref
26. Romeo V, Accardo G, Perillo T, et al. Assessment and
prediction of response to neoadjuvant chemotherapy in
breast cancer: a comparison of imaging modalities and
future perspectives. Cancers (Basel) 2021;13:3521. Crossref
27. Lafci O, Resch D, Santonocito A, Clauser P, Helbich T,
Baltzer PA. Role of imaging-based response assessment for
adapting neoadjuvant systemic therapy for breast cancer: a
systematic review. Eur J Radiol 2025:187:112105. Crossref
28. Caracciolo M, Castello A, Urso L, et al. Comparison of MRI
vs. [18F]FDG PET/CT for treatment response evaluation of
primary breast cancer after neoadjuvant chemotherapy:
literature review and future perspectives. J Clin Med
2023;12:5355. Crossref
29. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety
of neoadjuvant pertuzumab and trastuzumab in women
with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised
multicentre, open-label, phase 2 trial. Lancet Oncol
2012;13:25-32. Crossref
30. Hatschek T, Foukakis T, Bjöhle J, et al. Neoadjuvant
trastuzumab, pertuzumab, and docetaxel vs trastuzumab
emtansine in patients with ERBB2-positive breast
cancer: a phase 2 randomized clinical trial. JAMA Oncol
2021;7:1360-7. Crossref
31. Loibl S, André F, Bachelot T, et al. Early breast cancer:
ESMO Clinical Practice Guideline for diagnosis, treatment
and follow-up. Ann Oncol 2024;35:159-82. Crossref
32. National Library of Medicine, National Center for
Biotechnology Information, US. Comparison of axillary
lymph node dissection with axillary radiation for
patients with node-positive breast cancer treated with
chemotherapy. Available from: https://clinicaltrials.gov/study/NCT01901094. Accessed 13 Jan 2025.
33. Hyland CJ, Varghese F, Yau C, et al. Use of 18F-FDG PET/CT
as an initial staging procedure for stage II–III breast
cancer: a multicenter value analysis. J Natl Compr Canc
Netw 2020;18:1510-7. Crossref
34. Ming Y, Wu N, Qian T, et al. Progress and future trends in
PET/CT and PET/MRI molecular imaging approaches for
breast cancer. Front Oncol 2020;10:1301. Crossref
35. Zhang-Yin J. State of the art in 2022 PET/CT in breast
cancer: a review. J Clin Med 2023;12:968. Crossref