Hong Kong Med J 2025;31:Epub 10 Nov 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Progressive supranuclear palsy–like parkinsonism ensuing from anti–N-methyl-Daspartate receptor encephalitis
Yan Shen, MD, PhD; Chunyi Wang, MD, PhD; Ningyuan Wang, MD, PhD
Department of Neurology and Institute of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Corresponding author: Dr Yan Shen (shenyanzmins@sina.com)
An 18-year-old male postencephalitic patient was
admitted with a 2-year history of staggering gait,
bradykinesia, limb tremor, and memory decline
(online supplementary Fig). Two years previously,
he developed continuous fever, headache, psychosis,
and generalised seizures. Magnetic resonance
imaging scan at the time revealed remarkably high signals in the bilateral thalamus, midbrain and
hippocampus (Fig 1). Electroencephalography
showed diffuse slow waves, spikes and sharp
waves. Immunoelectrophoresis test determined a
type II oligoclonal band in the cerebrospinal fluid
(CSF). Antigen-specific cell-based assay detected
anti–N-methyl-D-aspartate receptor (NMDAR)
autoantibodies in the CSF (Fig 2). After exclusion
of potential pathogenic microbes and carcinomas,
a diagnosis of anti-NMDAR encephalitis was made.
The patient was prescribed immediate intravenous
immunoglobulin (0.4 g/kg/d for 5 days) and
methylprednisolone (500 mg/d, halved every 5
days). Perampanel (8 mg/d) was also administered
to control seizure attacks. His symptoms gradually
resolved and he was discharged 1 month later.
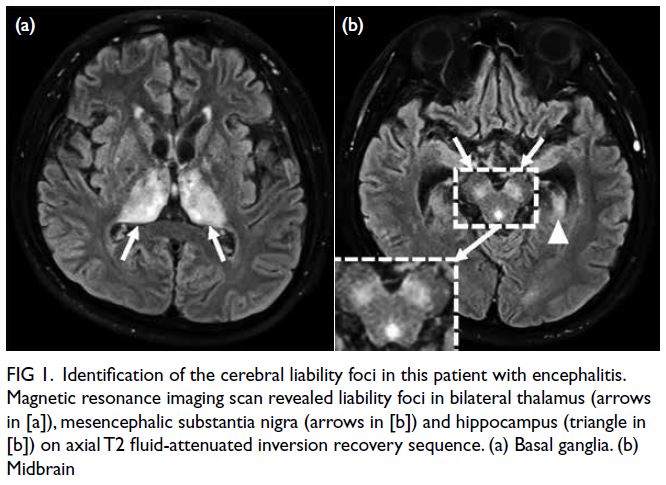
Figure 1. Identification of the cerebral liability foci in this patient with encephalitis. Magnetic resonance imaging scan revealed liability foci in bilateral thalamus (arrows in [a]), mesencephalic substantia nigra (arrows in [b]) and hippocampus (triangle in [b]) on axial T2 fluid-attenuated inversion recovery sequence. (a) Basal ganglia. (b) Midbrain
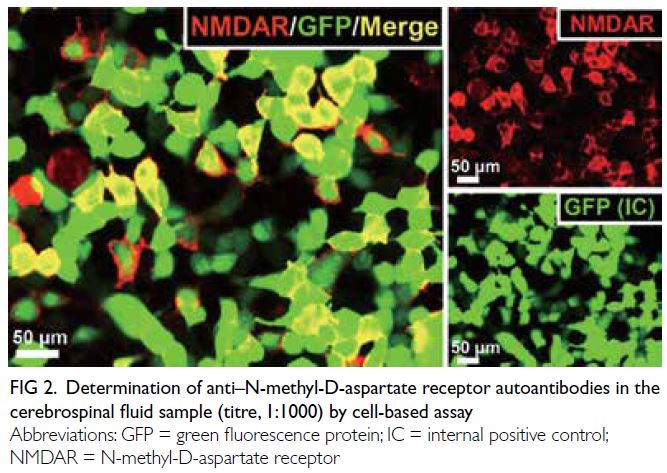
Figure 2. Determination of anti–N-methyl-D-aspartate receptor autoantibodies in the cerebrospinal fluid sample (titre, 1:1000) by cell-based assay
During the rehabilitation period, the patient
reported no relapse of encephalitis but presented
with insidious bradykinesia, limb tremor, unsteady
gait, and memory decline. These symptoms had
gradually worsened over the 2-year period and
contributed to frequent falls. He was wheelchair-bound
at admission. Physical examination revealed
limb tremor, hyperreflexia, patellar clonus, a positive
Babinski sign, and vertical supranuclear gaze palsy
(Fig 3). Mental status examination revealed space-time
disorientation. Magnetic resonance imaging
scan indicated remarkable midbrain atrophy (Fig 4). In contrast with the ‘convex’ contour before
the encephalitis (Fig 4a), the magnetic resonance
imaing scan in the postencephalitic stage revealed a
‘concave’ mesencephalic tegmental superior margin
and a decreased midbrain-to-pons axis ratio (Fig 4b), mimicking the characteristic ‘hummingbird
sign’ seen in progressive supranuclear palsy.
Antigen-specific cell-based assay of the CSF sample
determined a modest titre (in the ratio of 1: 10) of anti-NMDAR autoantibodies. A compound therapeutic
regimen of levodopa (750 mg/d), memantine (20
mg/d) and prednisone (60 mg/d) was initiated. At
3-month follow-up, the patient’s hypokinetic-rigid
and cognitive deficits had gradually resolved, and he
no longer required a wheelchair.
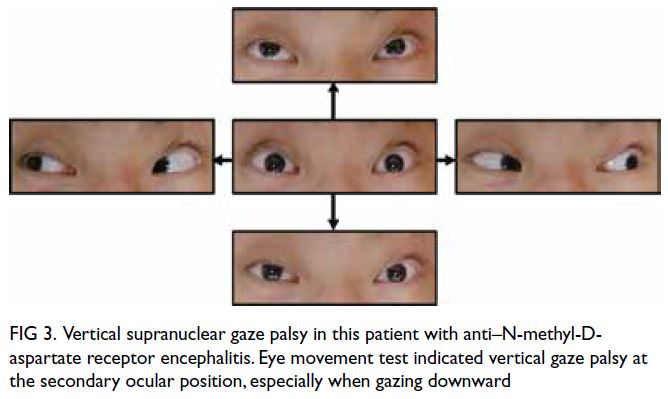
Figure 3. Vertical supranuclear gaze palsy in this patient with anti–N-methyl-Daspartate receptor encephalitis. Eye movement test indicated vertical gaze palsy at the secondary ocular position, especially when gazing downward
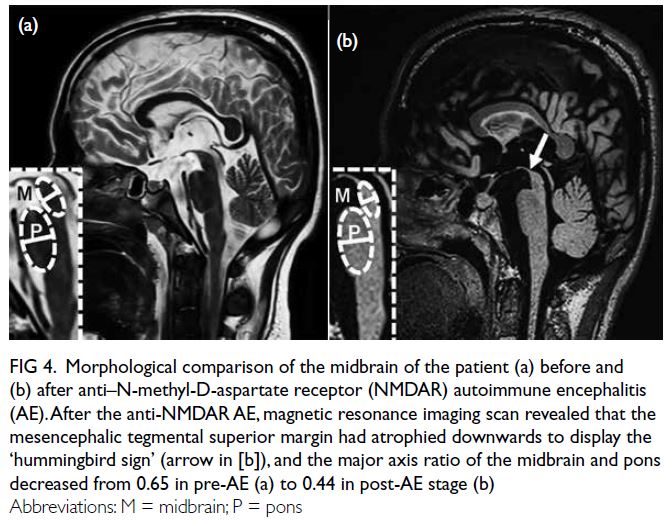
Figure 4. Morphological comparison of the midbrain of the patient (a) before and (b) after anti–N-methyl-D-aspartate receptor (NMDAR) autoimmune encephalitis (AE). After the anti-NMDAR AE, magnetic resonance imaging scan revealed that the mesencephalic tegmental superior margin had atrophied downwards to display the ‘hummingbird sign’ (arrow in [b]), and the major axis ratio of the midbrain and pons decreased from 0.65 in pre-AE (a) to 0.44 in post-AE stage (b)
Movement disorders are the third most
frequently observed symptom in anti-NMDAR
encephalitis.1 We reported the first case of progressive
supranuclear palsy–like parkinsonism consequent
to anti-NMDAR encephalitis. Intriguingly, the brain
regions implicated in this case coincided with the susceptible nuclei identified in parkinsonism.
Excitatory glutamatergic NMDAR subunits are
abundantly expressed on postsynaptic nigrostriatal
projection neurons, and are simultaneously under
the feedback modulation by dopaminergic afferents.2
Excessive glutamatergic activation, such as that seen
in anti-NMDAR encephalitis, can drive excitotoxic
neuronal death and contribute to progressive
Parkinsonian motor and cognitive deficits.3 A
previous study reported that co-morbidity of
anti-NMDAR encephalitis in Parkinson’s disease
worsens the existing extrapyramidal syndrome,
resulting in severe bradykinesia or even akinesia.4
Another recent study indicated that anti-NMDAR
autoantibodies correlated with worsening cognitive
deficits in Parkinson’s disease patients.5
Similarly, the modest titre of anti-NMDAR
antibody and amelioration of symptoms in this
case following prednisone treatment suggest
that persistent low-concentration autoantibody-mediated
excitotoxicity might underlie the
postencephalitic Parkinsonian and cognitive deficits,
although not induce clinical relapse of autoimmune
encephalitis. Nevertheless a proposed causal
relationship between autoimmune encephalitis
and postencephalitic neurodegeneration requires
clarification in future follow-up cohort studies.
Author contributions
Concept or design: Y Shen.
Acquisition of data: N Wang, C Wang.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: Y Shen.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: N Wang, C Wang.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: Y Shen.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study was supported by the National Natural Science
Foundation of China (Ref No.: 82301419) and the China
Postdoctoral Science Foundation (Ref No.: 2020M681442).
The funders had no role in study design, data collection/analysis/interpretation or manuscript preparation.
Ethics approval
This study was performed in accordance with the Declaration
of Helsinki. Informed consent was obtained from the patient
for all treatments and procedures, and for publication of this
article (including the clinical images).
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Morgan A, Li Y, Thompson NR, et al. Longitudinal
disability, cognitive impairment, and mood symptoms in
patients with anti-NMDA receptor encephalitis. Neurology
2024;102:e208019. Crossref
2. Ravenscroft P, Brotchie J. NMDA receptors in the basal
ganglia. J Anat 2000;196:577-85. Crossref
3. Campanelli F, Natale G, Marino G, Ghiglieri V, Calabresi P. Striatal glutamatergic hyperactivity in Parkinson’s disease. Neurobiol Dis 2022;168:105697. Crossref
4. Gastaldi M, Arbasino C, Dallocchio C, et al. NMDAR
encephalitis presenting as akinesia in a patient with
Parkinson disease. J Neuroimmunol 2019;328:35-7. Crossref
5. Gibson LL, Pollak TA, Hart M, et al. NMDA receptor
antibodies and neuropsychiatric symptoms in Parkinson’s
disease. J Neuropsychiatry Clin Neurosci 2023,35:236-43. Crossref

