Hong Kong Med J 2017 Aug;23(4):349–55 | Epub 28 Jun 2017
DOI: 10.12809/hkmj166030
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Transcatheter aortic valve implantation: initial experience in Hong Kong
Michael KY Lee, MB, BS, FRCP1; SF Chui, MB, ChB, FHKAM (Medicine)1; Alan KC Chan, MB, BS, FHKAM (Medicine)1;
Jason LK Chan, MB, BS, FHKAM (Medicine)1; Eric CY Wong, MB, BS, FHKAM (Medicine)1; KT Chan, MB, BS, FRCP1; HL Cheung, MB, ChB, FRCS2; CS Chiang, MB, BS, FRCP1
1 Department of Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Department of Cardiothoracic Surgery, Queen Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr Michael KY Lee (kylee1991@hotmail.com)
 A video clip showing transcatheter aortic valve implantation technique
A video clip showing transcatheter aortic valve implantation techniqueAbstract
Introduction: Aortic
stenosis is one of the most common valvular heart diseases in
the ageing population. Patients with symptomatic severe aortic
stenosis are at high risk of sudden death. Surgical
aortic-valve replacement is the gold standard of treatment but
many patients do not receive surgery because of advanced age
or co-morbidities. Recently, transcatheter aortic valve
implantation has been developed as an option for these
patients. This study aimed to assess efficacy and safety of
this procedure in the Hong Kong Chinese population.
Methods: Data for baseline patient characteristics,
procedure parameters, and clinical outcomes up to
1-year post-implantation in a regional hospital in
Hong Kong were collected and analysed.
Results: A total of 56 patients with severe aortic
stenosis underwent the procedure from December
2010 to September 2015. Their mean (± standard
deviation) age was 81.9 ± 4.8 years; 64.3% of them
were male. Their mean logistic EuroSCORE was
22.6% ± 13.4%. After implantation, the mean aortic
valve area improved from 0.70 cm2 ± 0.19 cm2 to 1.94 cm2 ± 0.37 cm2. Of the patients, 92%
were improved by at least one New York Heart
Association functional class. Stroke and major
vascular complications occurred in one (1.8%) and five (8.9%) patients, respectively. A permanent
pacemaker was implanted in seven (12.5%) patients.
Both hospital and 30-day mortalities were 1.8%.
The 1-year all-cause and cardiovascular mortality
rates were 12.5% and 7.1%, respectively.
Conclusions: Transcatheter aortic valve
implantation has been developed as an alternative
treatment for patients with symptomatic severe
aortic stenosis who are deemed inoperable or high
risk for surgery. Our results are very promising and
comparable with those of major clinical trials.
New knowledge added by this study
- Transcatheter aortic valve implantation (TAVI) is safe and feasible in patients with symptomatic severe aortic valve stenosis and high surgical risk.
- Clinical outcome was very promising for up to 1 year in patients who underwent TAVI.
- TAVI should be offered to patients with symptomatic severe aortic stenosis who are deemed inoperable or at high risk for open heart surgery.
Introduction
With improved living standards and advances in
medical treatment, the respective life expectancies
of males and females in Hong Kong have increased
from 72.3 years and 78.5 years in 1981, to 81.2 years
and 86.7 years in 2014.1 Aortic stenosis is one of the
most common valvular heart diseases in the ageing
population.2 The prevalence of aortic stenosis is up
to 4.6% in people older than 75 years.2 After onset of
symptoms, including the classic triad of chest pain,
heart failure or syncope, patients with severe aortic
stenosis are at very high risk of sudden death with
2-year mortality rate of up to 50% if left untreated.3 4 5 Surgical aortic-valve replacement (SAVR) is the gold
standard of treatment for patients with symptomatic
severe aortic stenosis.3 4 6 7 Many do not receive
surgical treatment, however, because of advanced
age or multiple co-morbidities.8 Transcatheter
aortic valve implantation (TAVI) has recently been
developed as an option for these patients who are
inoperable or at high risk of SAVR.9 10
Queen Elizabeth Hospital is the first hospital
in Hong Kong to perform TAVI since December
2010. This study aimed to assess the efficacy and
safety of this procedure in the Hong Kong Chinese
population.
Methods
In order to introduce TAVI into Hong Kong, a
Structural Heart Team comprising cardiologists,
cardiac surgeons, cardiac anaesthesiologists,
radiologists and cardiac nurses, was formed in early
2010 in Queen Elizabeth Hospital, which is a regional
hospital in Hong Kong. All potential patients
were assessed and interviewed independently
by cardiologists and cardiac surgeons. Clinical
assessment of functional status, transthoracic
echocardiogram, transoesophageal echocardiogram
(TEE), computed tomographic (CT) scan, and
conventional angiogram were performed according
to the protocol to assess the risks of SAVR and
suitability for TAVI. The Structural Heart Team
would undertake these investigations to assess
whether the risks of the patients were too high for
SAVR and if they were suitable for TAVI.
The correct size of the TAVI device was based
on the aortic annular dimensions measured by
TEE and CT scan. The preferred route of device
introduction was via the femoral artery. Based
on findings such as the vessel diameter, degree of
calcification and tortuosity found on CT imaging,
the subclavian artery or direct aortic approach was
also a valid alternative. The device was implanted
under fluoroscopic guidance and the correct
position monitored by fluoroscopy and TEE. Patients
underwent transthoracic echocardiogram prior
to discharge to assess device function and exclude
pericardial effusion. Post-discharge, regular clinic
visits, and serial transthoracic echocardiograms
were arranged to assess progress and monitor any
adverse events. All complications were reported to
an independent Safety Monitoring Committee of the
hospital.
The first TAVI device was used in December
2010 and was a self-expanding Medtronic CoreValve
device (Medtronic, Minneapolis [MN], US) [Fig 1a]. Subsequent to the introduction of its second-generation
Evolut R (Medtronic, Minneapolis [MN],
US) in 2015 (Fig 1b), it was used in most cases due
to its improved design of recapture/repositioning
ability and its smaller sheath size (18 Fr vs 14 Fr).
We obtained another self-expanding device with
recapture/repositioning ability (St Jude Medical
Portico; St Jude Medical, Minneapolis [MN], US;
Fig 1c), and a balloon-expandable device (Edwards
SAPIEN XT; Edwards Lifesciences, Irvine [CA], US) [Figs 1d and 1e] in 2015. This enhanced the ability to
treat a broad spectrum of patients with a wide variety
of clinical and anatomical challenges. The choice of
valve type was made by the Structural Heart Team,
based on the anatomy of the native aortic valve, size,
and calcification of iliofemoral vessels and need for
alternative access.
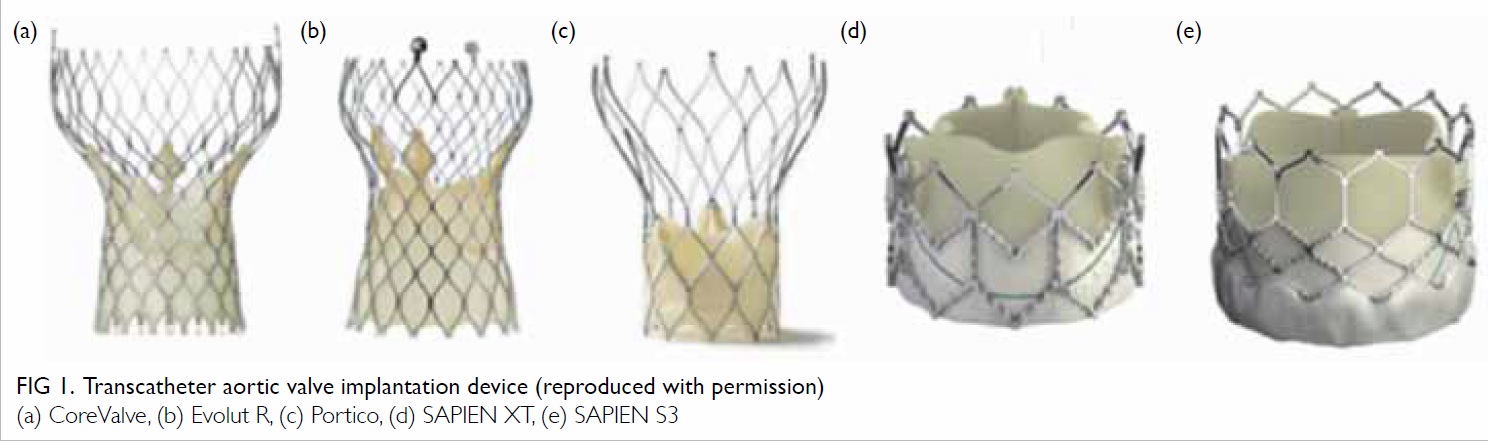
Figure 1. Transcatheter aortic valve implantation device (reproduced with permission)
(a) CoreValve, (b) Evolut R, (c) Portico, (d) SAPIEN XT, (e) SAPIEN S3
All patients who underwent TAVI during the
study period were entered into the TAVI registry
of our hospital. Their baseline characteristics,
procedural details, device used, and clinical
outcomes were recorded. They attended for regular
follow-up in our structural heart disease clinic as well
as regular echocardiographic monitoring. Follow-up
data were also added to the registry. Any patient who
defaulted follow-up was contacted; if they had died,
cause of death was retrieved from their electronic
patient record of Hospital Authority of Hong
Kong.
We retrieved and analysed the data of the
TAVI registry. Descriptive statistics were used to
report baseline characteristics, procedural results,
and clinical outcomes. Analysis was performed
using the Microsoft Office Excel (Mac version 2011;
Microsoft, Washington, US). The study was performed in accordance with the principles outlined in the Declaration of Helsinki.
Results
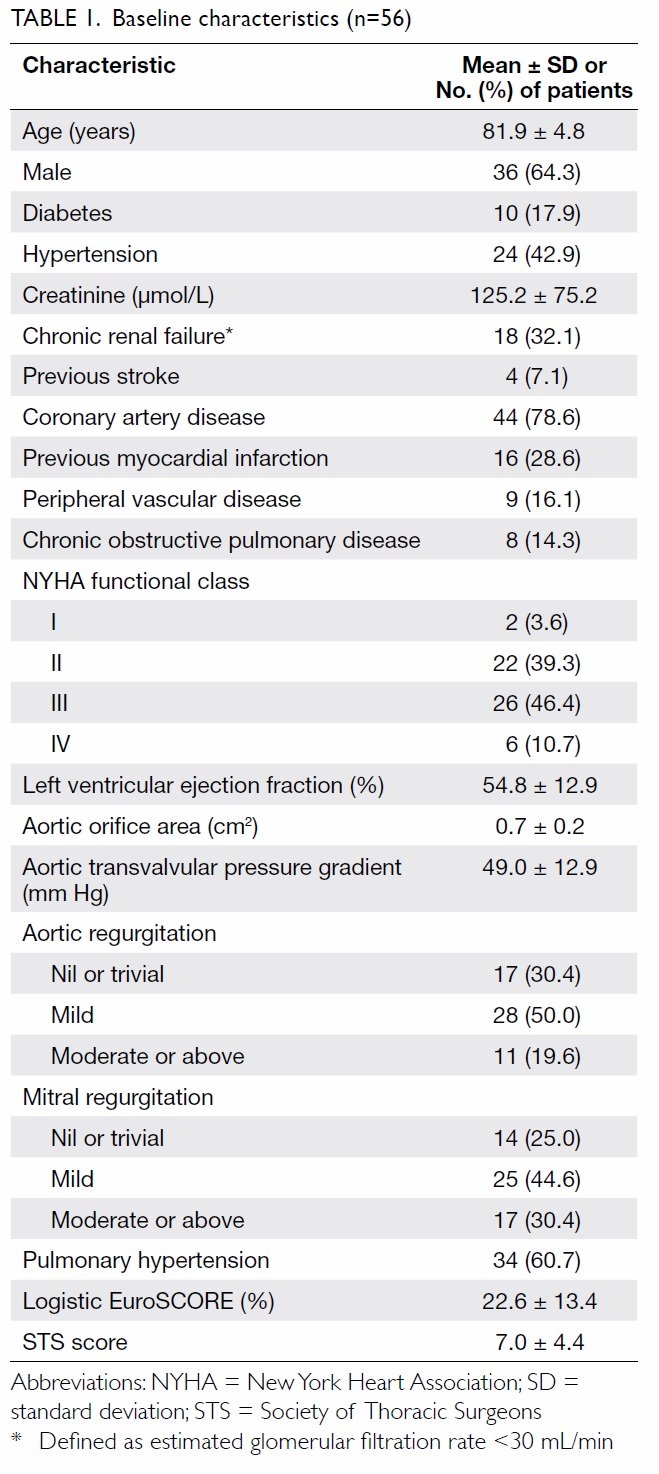
Baseline characteristics
From December 2010 to September 2015, a total of
56 patients with symptomatic severe aortic stenosis
underwent TAVI. Their baseline characteristics are
outlined in Table 1. Their mean (± standard deviation)
age was 81.9 ± 4.8 years, and the majority (64.3%) were male. The prevalence of severe co-morbidities was
predicted as high, with a mean logistic EuroSCORE
of 22.6% ± 13.4% and a mean Society of Thoracic
Surgeons score of 7.0 ± 4.4. Several variables were
taken into account when calculating these risk scores,
such as age, symptoms at presentation, current
haemodynamic status, left ventricular systolic
function, baseline renal function, New York Heart
Association (NYHA) functional class, and presence
of other co-morbidities such as peripheral artery
disease, pulmonary disease, and cerebrovascular
disease. The mean left ventricular ejection fraction
(LVEF) was 54.8% ± 12.9%. Most patients had
various degrees of heart failure symptoms with 22
(39.3%), 26 (46.4%), and six (10.7%) patients in NYHA
class II, III, and IV, respectively. All patients had
severe aortic stenosis on echocardiography, defined
by standard criteria with a mean aortic valve area
of 0.7 cm2 ± 0.2 cm2 and mean aortic transvalvular pressure gradient of 49.0 mm Hg ± 12.9 mm Hg.
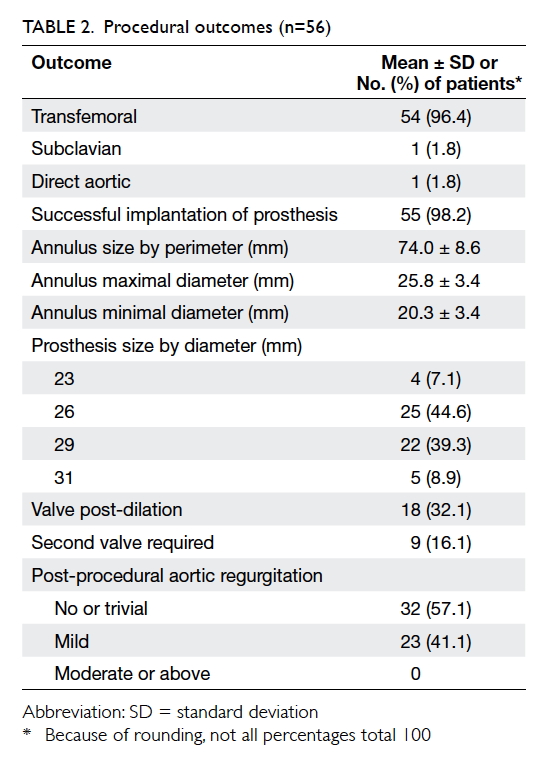
Procedural outcomes
The procedural outcomes are summarised in Table 2. Successful implantation was completed in 98.2% of patients and all procedures were performed
under general anaesthesia. Transfemoral access
was successful in most cases (54 patients, 96.4%)
although one (1.8%) patient was treated via the
subclavian approach and one (1.8%) via a direct
aortic approach. The most commonly used TAVI
device was a 26-mm prosthesis. A second valve was
required during the index procedure in nine (16.1%)
patients due to suboptimal position of the first
device. Most patients (32/56, 57.1%) had no or
trivial aortic regurgitation following implantation
and 23 (41.1%) had mild aortic regurgitation. None
had moderate or severe aortic regurgitation.
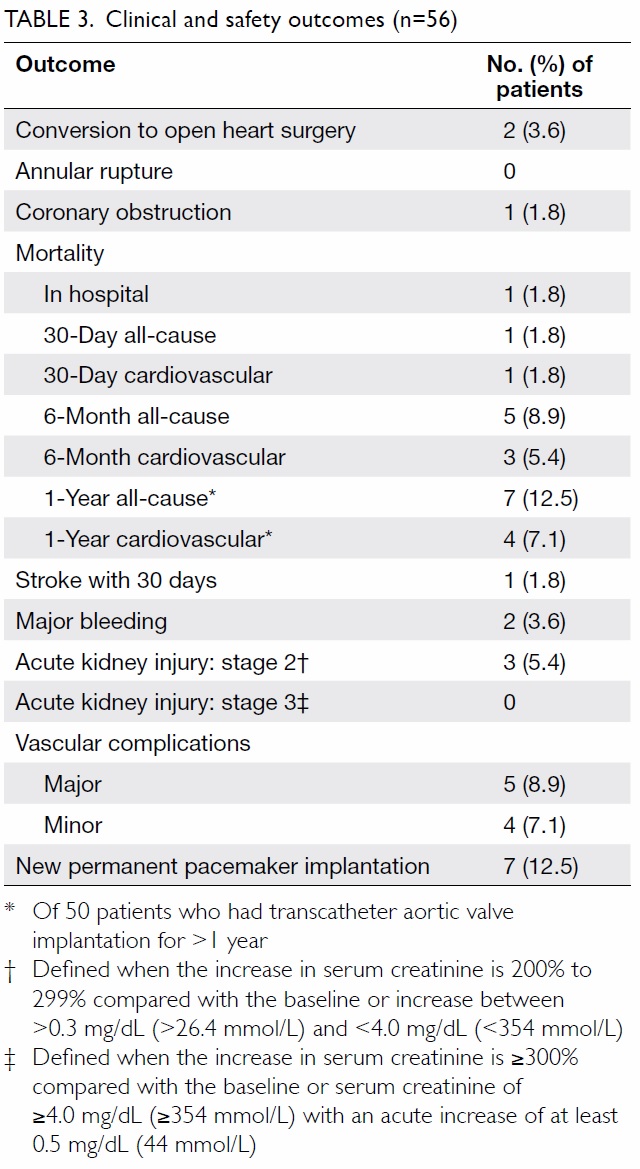
Adverse events
The safety endpoints and clinical outcomes are
outlined in Table 3. Conversion to open heart
surgery after the procedure was necessary in two
patients, one of whom had a calcified valvular leaflet
that dislodged into the left atrium after device
implantation and required open exploration. The
other patient had incessant ventricular fibrillation,
possibly due to coronary obstruction during the
procedure, and required emergent cardiopulmonary
bypass and open heart surgery. Pre-procedural
CT revealed adequate coronary height (both left
coronary and right coronary ostium >16 mm above
annular plane) and adequate sinus of Valsalva
diameters. The coronary obstruction was thought to
be due to dislodged calcified nodules from the aortic
valve leaflets. This patient eventually died despite
SAVR under extracorporeal membrane oxygenation
support, and was the only hospital mortality
(1.8%).
Stroke occurred in one (1.8%) patient within
30 days. New conduction abnormalities requiring
permanent pacing were present in seven (12.5%)
patients. There were major access-related vascular
complications in five (8.9%) patients and three
(5.4%) had acute kidney injury stage 2 although none
required long-term dialysis and no patient had acute
kidney injury stage 3 (Table 3).
Follow-up results
Most patients (55/56, 98.2%) either had regular
follow-up or died. Only one patient who relocated
to Mainland China was lost to follow-up, although
his doctor keeps us updated regularly about his
condition. The longest follow-up period was 5
years. The 30-day mortality rate was 1.8%. The
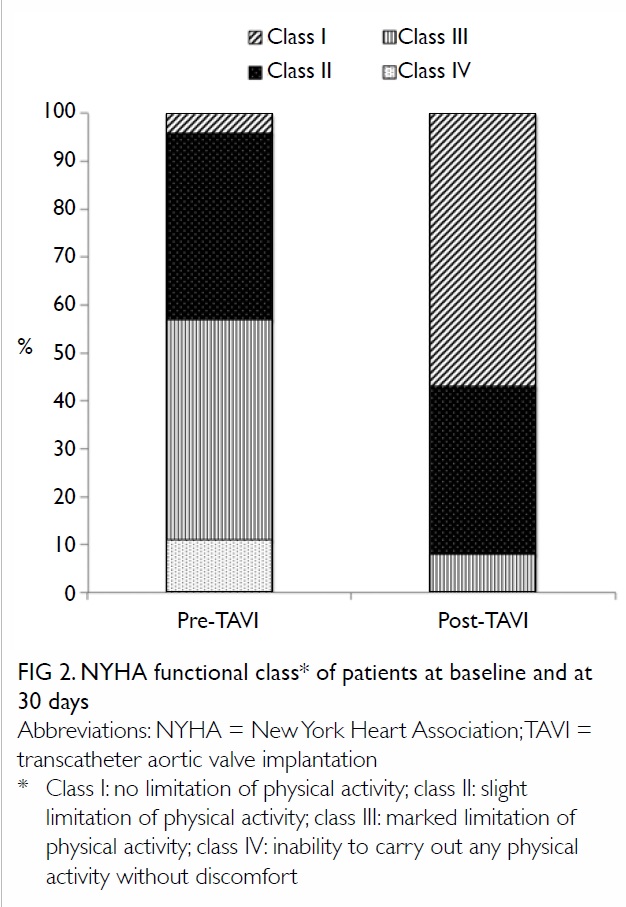
NYHA functional class of patients at baseline and
at 30 days is outlined in Figure 2; 92% of patients
improved by at least one functional class. The mean
LVEF was 57.9% ± 10.9% at 30 days. The mean
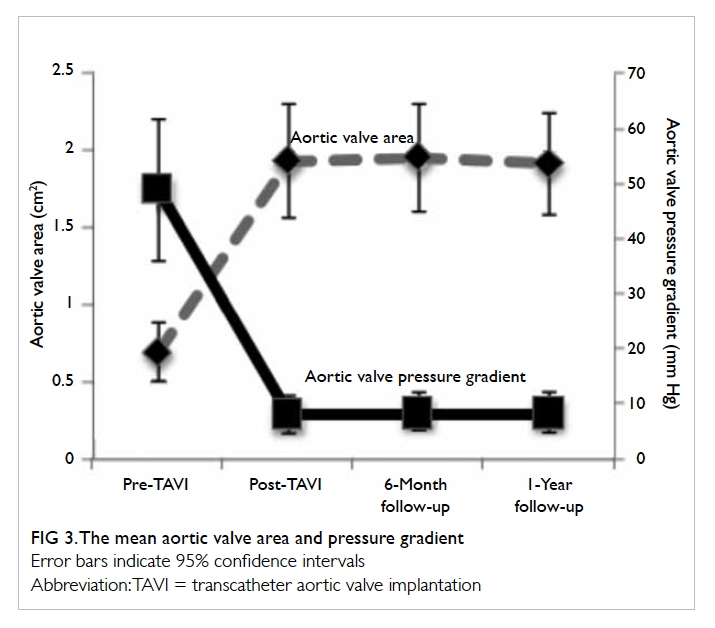
aortic valve area improved to 1.94 cm2 ± 0.37 cm2
and mean aortic transvalvular pressure gradient
improved to 8.1 mm Hg ± 3.4 mm Hg (Fig 3). These
improvements persisted at 6-month and 1-year
follow-up assessment. The 6-month all-cause and
cardiovascular mortalities were 8.9% and 5.4%,
respectively. The 1-year all-cause and cardiovascular
mortalities were 12.5% and 7.1%, respectively (Table 3). Causes of 1-year mortality included myocardial
infarction, heart failure with cardiogenic shock, liver failure, pneumonia, and sepsis with disseminated
intravascular coagulopathy.
Discussion
This is the first report to describe the clinical
experience of TAVI in a major tertiary referral
hospital, which is also the first hospital in Hong
Kong to introduce this technology and having the
largest case volume in Hong Kong up until the end
of 2015. The clinical outcomes of our cohort show
very promising results.
Since 2010, there have been several
landmark clinical trials describing the initial
clinical outcome of TAVI in both high-surgical-risk
and inoperable patients. The PARTNER 1 was
a multicentre prospective randomised controlled
trial to investigate the balloon-expandable Edwards
SAPIEN valve (Edwards Lifesciences). PARTNER
1B arm randomised inoperable patients to TAVI or
best medical treatment and showed a 20% absolute
reduction in mortality rate at 1 year with TAVI.9
The PARTNER 1A arm randomised patients with
high surgical risk to either TAVI or SAVR and TAVI
was shown to be non-inferior to SAVR in terms of
mortality rate at 1 year (24% vs 26.8%; P=0.44).11
The US CoreValve pivotal trial randomised patients
with high surgical risk to either TAVI using the self-expanding
CoreValve or SAVR and demonstrated a
significant reduction in mortality rate at 1 year in
the TAVI group compared with the SAVR group
(14.2% vs 19.1%; P=0.04).10 The relatively high 1-year
mortality rate in those clinical trials is mainly due
to their baseline multiple co-morbidities and the
advanced age (mean age, 80 years) of patients.
The 1-year mortality rate of 12.5% in our cohort
compares well with these landmark trials. The risk
profile of our patient group is nonetheless not lower
(logistic EuroSCORE of 22% in our group vs 18%
in CoreValve pivotal trial). This high baseline risk
factor accounts for the high 1-year mortality rate
although a significant proportion was related to
non-cardiac causes (3 out of 7). The current view is
to avoid treating the patients with too high risk and
who will not benefit from this high-risk procedure.
A reasonable 1-year expected survival is also a
prerequisite.12 13
Other complication rates of our cohort also
compared well with the landmark trials. Significant
aortic regurgitation secondary to paravalvular
leak (PVL) is a unique problem not uncommonly
encountered following TAVI in contrast to patients
who undergo SAVR in whom there is usually no
residual leakage.14 15 One study showed that even mild
PVL after TAVI is associated with higher mortality
rate at 2 years.16 Treatment of significant PVL is by
post-dilation, putting in another valve (so called
‘valve in valve’) or using a vascular plug. The rate of
moderate or severe PVL in both the PARTNER 1B9 and US CoreValve pivotal trial10 was approximately
7%. No moderate or severe PVL was noted in our study due to the
high rate of using two valves (‘valve in valve’) in 16%
of our cohort compared with the reported 4% rate
in the US CoreValve pivotal trial. With increasing
experience and availability of a repositionable
device, however, progressively fewer cases required
more than one valve.
Some of the complications after TAVI are
more disabling than others, such as stroke. The
major stroke rate in both the PARTNER and US
CoreValve pivotal trial was approximately 5%.9 10 Major stroke rate in one meta-analysis of more
than 10 000 patients was 3.3%.17 Clinical stroke
occurred in only one (1.8%) patient in our cohort.
This is probably because of our small sample size, as
well as the random and unpredictable nature of this
complication. More cases of stroke would be expected
if more patients were treated. The latest clinical
trial using next-generation TAVI devices resulted
in a much lower rate of stroke after TAVI (0.9% at
30 days and 2.4% at 1 year for high-risk patients).18
The aetiology of stroke after TAVI is multifactorial
and includes embolism of valvular material during
balloon valvuloplasty, device manipulation across an
atheromatous aorta, and atrial fibrillation.19 Multiple
strategies to reduce periprocedural stroke have
been attempted including direct stenting, avoidance
of pre- or post-dilation, use of cerebral protection
devices and different antithrombotic regimens.20 21
Currently, randomised trials are underway to
determine whether cerebral protection devices are
useful in reducing periprocedural stroke.22
Conduction abnormality is another common
event following TAVI. The reported rate of
permanent pacemaker implantation to treat high-grade
heart block varies from 10% to 30% and
it depends very much on type and implantation
depth of the device.23 24 25 26 The rate reported in the US
CoreValve pivotal trial was 20% at 1 month and 22%
at 1 year.10 The permanent pacemaker implantation
rate in our cohort was 12.5% with the majority of our
cases having a self-expandable valve. In our cohort,
most pacemakers were implanted earlier on in the
study period when we were more cautious about
treating post-procedural conduction abnormalities.
Major vascular complications occurred in
approximately 6% of patients in the US CoreValve
pivotal trial and 11% in PARTNER 1 trial.9 10 Rates
of major vascular complications in different
observational and randomised trials range from 5%
to 17%.27 The lower rate in the US CoreValve pivotal
trial can be explained by the smaller size of the
introducer sheath for CoreValve (18 Fr) compared
with the much bigger 22-24 Fr sheath for the first-generation
SAPIEN device in the PARTNER 1 trial.9 For the same reason and the relative smaller size of
peripheral vessels in an Asian population, we would
expect a higher rate of vascular complications.
Indeed, the major vascular complication rate in
our cohort was 8.9%, which is compatible with
the worldwide standard. This is the result of our
comprehensive use of CT angiogram for all cases
from the beginning of the cohort to delineate the
size of the peripheral vessels and better plan of
procedural strategies.
Overall, the success of the procedure depends
not only on the technical requirement in a very high-risk
group of patients but also a comprehensive,
multidisciplinary team approach. This ‘heart
team’ approach is the cornerstone of the rapidly
developing field of structural heart intervention
and preferred strategies in dealing with anticipated
complications.27
After the success in treating high-risk
patients with aortic stenosis, the recently published
PARTNER 2 trial evaluated TAVI and SAVR
in patients with intermediate surgical risk. It
randomised patients with intermediate surgical risk
to either TAVI or SAVR; TAVI was non-inferior to
SAVR in terms of all-cause mortality and disabling
stroke at 2 years (19.3% in TAVI group vs 21.1% in
SAVR group; P=0.25).28 Another major trial testing
TAVI in intermediate-risk patients, the SURTAVI
Trial (ClinicalTrials.gov number NCT01586910),
has completed patient recruitment and the results
will be available very soon.
Limitations
The limitations of the current study include the
relatively small sample size and a single-centre early
experience. The technology is evolving and lower- or
intermediate-risk patients are being treated in
various clinical trials. With an increasing awareness
of the disease and referrals from around the territory,
the population being treated is expected to increase
in the coming years.
Conclusions
The technique TAVI has been developed as an
alternative treatment for patients with symptomatic
severe aortic stenosis who are deemed inoperable
or high risk for surgery. It has been proven in major
randomised controlled trials to have an acceptable
complication rate and durability in the medium
term. Our results are very promising and comparable
with those of major clinical trials. Long-term clinical
outcomes should be diligently monitored.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Women and men in Hong Kong—key statistics. 2015
Edition. Available from: http://www.statistics.gov.hk/pub/B11303032015AN15B0100.pdf. Accessed 26 Jun 2016.
2. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott
CG, Enriquez-Sarano M. Burden of valvular heart diseases:
a population-based study. Lancet 2006;368:1005-11. Crossref
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of
Cardiology/American Heart Association Task Force on
Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. Crossref
4. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on
the management of valvular heart disease (version 2012):
the joint task force on the management of valvular heart
disease of the European Society of Cardiology (ESC) and
the European Association for Cardio-Thoracic Surgery
(EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. Crossref
5. Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous
course of aortic valve disease. Eur Heart J 1987;8:471-83. Crossref
6. Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am Coll
Cardiol 2000;35:747-56. Crossref
7. Kvidal P, Bergström R, Malm T, Ståhle E. Long-term
follow-up of morbidity and mortality after aortic valve
replacement with a mechanical valve prosthesis. Eur Heart
J 2000;21:1099-111. Crossref
8. Bouma BJ, van Den Brink RB, van der Meulen JH, et al. To
operate or not on elderly patients with aortic stenosis: the
decision and its consequences. Heart 1999;82:143-8. Crossref
9. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve
implantation for aortic stenosis in patients who
cannot undergo surgery. N Engl J Med 2010;363:1597-607. Crossref
10. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter
aortic-valve replacement with a self-expanding prosthesis.
N Engl J Med 2014;370:1790-8. Crossref
11. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus
surgical aortic-valve replacement in high-risk patients. N
Engl J Med 2011;364:2187-98. Crossref
12. Miller DC. TAVI has a limited role in the treatment of AS.
American Association of Thoracic Surgery 2012 Annual
Meeting; 2012 Apr 29; San Francisco, United States.
13. Gilard M, Eltchaninoff H, Lung B, et al. Registry of
transcatheter aortic-valve implantation in high-risk
patients. N Engl J Med 2012;366;1705-15. Crossref
14. Lerakis S, Hayek SS, Douglas PS. Paravalvular aortic leak
after transcatheter aortic valve replacement: current
knowledge. Circulation 2013;127:397-407. Crossref
15. Généreux P, Head SJ, Hahn R, et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles’ heel? A comprehensive review of the literature. J Am Coll Cardiol 2013;61:1125-36. Crossref
16. Kodali SK, Williams MR, Smith CR, et al. Two-year
outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. Crossref
17. Eggebrecht H, Schmermund A, Voigtländer T, Kahlert
P, Erbel R, Mehta RH. Risk of stroke after transcatheter
aortic valve implantation (TAVI): a meta-analysis of 10,037
published patients. EuroIntervention 2012;8:129-38. Crossref
18. Herrmann HC. SAPIEN 3: Evaluation of a balloon-expandable
transcatheter aortic valve in high-risk and
inoperable patients with aortic stenosis with aortic TCT.
San Francisco 2015 Oct 15.
19. Mastoris I, Schoos MM, Dangas GD, Mehran R. Stroke
after transcatheter aortic valve replacement: incidence,
risk factors, prognosis, and preventive strategies. Clin
Cardiol 2014;37:756-64. Crossref
20. Ghanem A, Naderi AS, Frerker C, Nickenig G, Kuck
KH. Mechanisms and prevention of TAVI-related
cerebrovascular events. Curr Pharm Des 2016;22:1879-87. Crossref
21. Schmidt T, Schlüter M, Alessandrini H, et al. Histology
of debris captured by a cerebral protection system
during transcatheter valve-in-valve implantation. Heart
2016;102:1573-80. Crossref
22. Kodali S. Cerebral embolic protection devices: Sentinel
dual filter device. Update of US Pivotal Clinical Trial. TCT
2015; 2015 Oct 11-15; San Francisco, United States.
23. Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic
valve implantation: impact on clinical and valve-related
outcomes. Circulation 2009;119:3009-16. Crossref
24. Piazza N, Grube E, Gerckens U, et al. Procedural and
30-day outcomes following transcatheter aortic valve
implantation using the third generation (18 Fr) corevalve
revalving system: results from the multicentre, expanded
evaluation registry 1-year following CE mark approval.
EuroIntervention 2008;4:242-9. Crossref
25. Jilaihawi H, Chin D, Vasa-Nicotera M, et al. Predictors for
permanent pacemaker requirement after transcatheter
aortic valve implantation with the CoreValve bioprosthesis.
Am Heart J 2009;157:860-6. Crossref
26. Kanmanthareddy A, Buddam A, Sharma S, et al. Complete
heart block after transcatheter aortic valve implantation: a
meta-analysis. Circulation 2014;130:A15832.
27. Toggweiler S, Leipsic J, Binder RK, et al. Management of
vascular access in transcatheter aortic valve replacement:
part 2: Vascular complications. JACC Cardiovasc Interv
2013;6:767-76. Crossref
28. Leon MB, Smith CR, Mack M, et al. Transcatheter or
surgical aortic-valve replacement in intermediate-risk
patients. N Engl J Med 2016;374:1609-20. Crossref